Eli Lilly and Company (NYSE:LLY) announced that the FDA has granted a priority review to the supplemental biologics license application (sBLA) for its calcitonin gene-related peptide (CGRP) antibody, Emgality (galcanezumab). With this latest sBLA filing, Lilly is looking to get Emgality injection approved for the preventive treatment of episodic cluster headache in adult patients.
Notably, Lilly gained an FDA approval for Emgality, its CGRP antibody, for adult migraine in late last September.
Generally, the FDA provides a priority review designation to drugs with potential for driving significant improvements in the safety and effectiveness of treatment, prevention or diagnosis of a serious disease.
The sBLA filing was based on data from a phase III study, which evaluated the safety and efficacy of Emgality injection (300mg) in adult patients with episodic cluster headache. Last September, the FDA granted a Breakthrough Therapy status to Emgality for the same indication. Currently, there are no approved medicines in the United States for treating episodic cluster headache. Thus, a nod will help Lilly gain access to a broader patient population and boost sales for this promising drug.
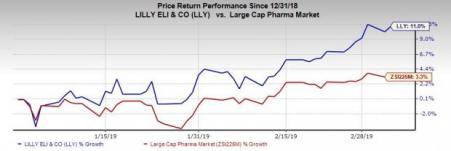
Shares of Lilly have rallied 11% so far this year compared with the industry’s increase of 3.3%.
Emgality was the third CGRP antibody to get an FDA nod in 2018 for migraine treatment. Amgen’s (NASDAQ:AMGN) and Novartis’ CGRP antibody, Aimovig, was approved and launched in the United States during the second quarter of 2018 while Teva’s (NYSE:TEVA) Ajovy was also approved last September.
Lilly is also enrolling patients in phase III studies on Emgality for the preventive treatment of migraine in children and adolescents, aged 6-17 years.
Notably, Lilly’s overall pain portfolio looks promising. Apart from Emgality, the company is developing lasmiditan, which is currently under review in the United States for the treatment of acute migraine in adult patients. Lilly along with partner Pfizer (NYSE:PFE) is developing tanezumab, which is being evaluated in several phase III programs for treating osteoarthritis pain (OA), chronic low back pain and cancer pain in adult patients.
Zacks Rank
Lilly currently carries a Zacks Rank #4 (Sell).
You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Today's Best Stocks from Zacks
Would you like to see the updated picks from our best market-beating strategies? From 2017 through 2018, while the S&P 500 gained +15.8%, five of our screens returned +38.0%, +61.3%, +61.6%, +68.1%, and +98.3%.
This outperformance has not just been a recent phenomenon. From 2000 – 2018, while the S&P averaged +4.8% per year, our top strategies averaged up to +56.2% per year.
See their latest picks free >>
Pfizer Inc. (PFE): Free Stock Analysis Report
Eli Lilly and Company (LLY): Free Stock Analysis Report
Amgen Inc. (AMGN): Free Stock Analysis Report
Teva Pharmaceutical Industries Ltd. (TEVA): Free Stock Analysis Report
Original post
Zacks Investment Research
