Amgen Inc. (NASDAQ:AMGN) and partner UCB SA UCBJF announced that the FDA has issued a complete response letter (CRL) for the biologics license application (BLA) for Evenity (romosozumab). Amgen is looking to get Evenity approved for the treatment of postmenopausal women with osteoporosis.
The original BLA included data from the pivotal FRAME study.
However, the FDA now requires safety and efficacy data from the other two pivotal phase III studies - ARCH and BRIDGE - to be included in the application. The agency has asked for a resubmission of the BLA, which will be considered as an extension of the current review.
We remind investors that in May, Amgen had presented data from ARCH fracture study of romosozumab compared to Merck & Co., Inc.’s (NYSE:MRK) Fosamax (alendronate) in postmenopausal women with osteoporosis. Data from the study showed that Evenity/romosozumab led to a statistically significant superior fracture risk reduction than Fosamax (alendronate), which is the current standard of care in osteoporosis. However, a cardiovascular side effect was observed in the study.
The company then said that the safety data from the ARCH data will now be considered in the regulatory review as agreed with the FDA. Following the heart-related safety issue that emerged from the otherwise successful ARCH results, Amgen warned that it did not expect Evenity to be approved this year.
The CRL, thus, does not come as a surprise.
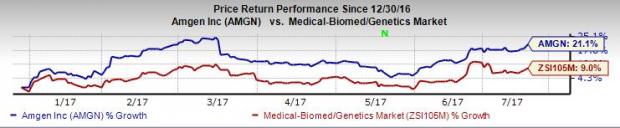
So far this year, Amgen’s shares are up 21.1%, better than the 9.0% increase registered by the Zacks categorized Biomed/Genetics industry.
The FRAME study evaluated the effectiveness of Evenity in reducing the risk of new vertebral fractures over 12 months. It also compared the effectiveness of 12 months of Evenity administration, followed by another 12 months of Xgeva to placebo followed by Xgeva administration.
The BRIDGE study evaluated Evenity in older men with osteoporosis and a history of fragility fracture or vertebral fracture. The study evaluated the effectiveness of Evenity in increasing bone mineral density (BMD) at the lumbar spine and its effect on BMD at the femoral neck and total hip.
The company is working along with the FDA and is in a process to re-submit the BLA with the requested data.
In a separate press release, Amgen announced the submission of a supplemental New Drug Application (sNDA) to the FDA and a variation to the marketing application to the European Medicines Agency (EMA) for its myeloma drug, Kyprolis. The submission of the regulatory applications was done to expand the drug’s label to include overall survival (OS) data from phase III ENDEAVOR study.
We remind investors that Kyprolis (twice weekly) is already approved for use in combination with dexamethasone or with Celgene Corporations’ (NASDAQ:CELG) Revlimid (lenalidomide) plus dexamethasone based on the primary analysis of progression-free survival (PFS) data from the ASPIRE study.
The submissions include data from the pivotal ENDEAVOR study, which compared Kyprolis and J&J’s Velcade in combination with dexamethasone in patients with relapsed or refractory multiple myeloma. The data demonstrated that Kyprolis reduced the risk of death by 21% compared to Velcade and increased the median OS by 7.6 months (47.6 months vs. 40 months).
Kyprolis is the first and only therapy to achieve superior OS in a head-to-head comparison with a current standard of care, Velcade.
Last week, Amgen announced OS data from the ASPIRE study. Overall survival data from the study showed that patients with relapsed or refractory multiple myeloma lived 7.9 months longer when treated with Kyprolis plus Revlimid and dexamethasone (KRd) compared to those treated with Revlimid and dexamethasone (Rd) (median OS of 48.3 months for KRd versus median OS of 40.4 months for Rd).).
Kyprolis is also being evaluated in a couple of other studies for potential label expansion. Amgen is evaluating a weekly dosing regimen of Kyprolis in a phase III study (ARROW) in relapsed and refractory multiple myeloma patients. Data is expected to be out in 2019.
Zacks Rank
Amgen currently has a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Today's Stocks from Zacks' Hottest Strategies
It's hard to believe, even for us at Zacks. But while the market gained +18.8% from 2016 - Q1 2017, our top stock-picking screens have returned +157.0%, +128.0%, +97.8%, +94.7%, and +90.2% respectively.
And this outperformance has not just been a recent phenomenon. Over the years it has been remarkably consistent. From 2000 - Q1 2017, the composite yearly average gain for these strategies has beaten the market more than 11X over. Maybe even more remarkable is the fact that we're willing to share their latest stocks with you without cost or obligation. See Them Free>>
Merck & Company, Inc. (MRK): Free Stock Analysis Report
Celgene Corporation (CELG): Free Stock Analysis Report
Amgen Inc. (AMGN): Free Stock Analysis Report
UCB SA (UCBJF): Free Stock Analysis Report
Original post
Zacks Investment Research