Biotechnology company, Regeneron (NASDAQ:REGN) Pharmaceuticals REGN, focuses on the discovery, development, and commercialization of treatments targeting serious medical conditions.
Favorable Rank and Rising Estimates: The company has delivered a strong performance in 2021 on the back of a solid diverse portfolio. It currently sports a Zacks Rank #1 (Strong Buy). You can see the complete list of today’s Zacks #1 Rank stocks here.
Earnings per share estimates have increased to $61.41 from $53.22 for 2021 and to $46.73 from $44.11 for 2022 in the past 30 days.
Regeneron’s shares have gained 14.5% so far this year against the industry’s decline of 12.2%.
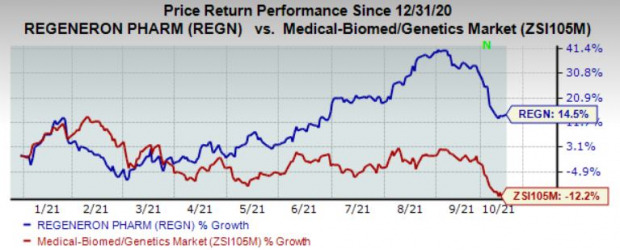 Image Source: Zacks Investment Research
Image Source: Zacks Investment Research
Lead Drug Eylea Drives Growth: Demand for lead ophthalmology drug, Eylea, (approved for neovascular age-related macular degeneration, diabetic macular edema, and macular edema, among others) revived after a slowdown last year. Regeneron has a collaboration agreement with Bayer (OTC:BAYRY) BAYRY for Eylea. Recent label expansions into additional indications have boosted sales and should maintain momentum for the drug despite lingering competition. The company is working to expand the drug’s label further, which should boost performance.
Dupixent Maintains Stellar Performance: Regeneron has a collaboration agreement with Sanofi (NASDAQ:SNY) SNY for some of its other drugs like Dupixent. Strong performance of Dupixent has driven growth for Regeneron in the last few quarters. The drug is approved for moderate-to-severe atopic dermatitis (AD) and asthma, among others. Continued label expansion of the drug has boosted sales further, making it an important sales contributor for Regeneron. The FDA also approved the drug as an add-on maintenance therapy in patients aged 12 years or older with moderate-to-severe asthma with an eosinophilic phenotype or oral corticosteroid-dependent asthma and an add-on maintenance treatment for adults with inadequately-controlled severe chronic rhinosinusitis with nasal polyps (CRSwNP). Regeneron records its share of profits/losses in connection with global sales of Dupixent and Kevzara.
New Drug Approvals Diversify Revenue Base: We are impressed by Regeneron’s efforts to bring additional products to the market. The FDA approval of Libtayo for the treatment of patients with metastatic or locally advanced cutaneous squamous cell carcinoma (CSCC) who are not candidates for curative surgery or curative radiation has diversified the portfolio. The drug’s label recently expanded to include non-small cell lung cancer (NSCLC) and basal cell carcinoma (BCC). It is under review for the indication of cervical cancer in the United States. Approval in additional indications will boost sales further as the oncology market holds great potential.
The FDA approvals of Inmazeb (REGN-EB3) for the treatment of infection caused by Zaire ebolavirus in adult and pediatric patients, including newborns of mothers who have tested positive for the infection and Evkeeza for the treatment of adults and adolescents with HoFH add an incremental stream of revenues for the company. Moreover, these drugs diversify the company’s revenue base.
Antibody Cocktail Boosts Top Line: Regeneron has had a stupendous year so far on the incremental contribution from REGEN-COV, its antibody cocktail for COVID-19. REGEN-COV is an investigational medicine authorized by the FDA under an emergency use authorization (EUA) to treat people who are at high risk of serious consequences from COVID-19 infection. They are either already infected or are in certain post-exposure prophylaxis settings. However, it is not authorized for patients who are hospitalized due to COVID-19 infection in the United States. The FDA recently updated the EUA for REGEN-COV, lowering the dose to 1,200 mg (600 mg casirivimab and 600 mg imdevimab), which is half the dose originally authorized. Regeneron is in discussions with the FDA to expand the current EUA to other populations, including prevention and hospitalized patient settings. The FDA recently updated the EUA for REGEN-COV, lowering the dose to 1,200 mg (600 mg casirivimab and 600 mg imdevimab), which is half the dose originally authorized. Regeneron is in discussions with the FDA to expand the current EUA to other populations, including prevention and hospitalized patient settings.
Incremental contribution from REGEN-COV has boosted the top line as the pandemic wreaks havoc with emerging deadly variants that question the efficacy of the vaccines. The company recently announced that the U.S. Department of Health and Human Services and the Department of Defense will purchase 1.4 million additional doses of REGEN-COV.
In August, Regeneron submitted the first of two biologics license applications (BLAs) for REGEN-COV. The initial submission included data on the efficacy and safety of REGEN-COV to treat and prevent SARS-CoV-2 infection in non-hospitalized people. The second BLA submission will focus on those hospitalized because of COVID-19, and is expected to be completed later this year. It has a collaboration agreement with Roche RHHBY (OTC:RHHBY) for the same.
In brief, Regeneron has a solid and diverse portfolio, which is expected to maintain the company’s momentum further despite stiff competition for its key drugs. The promising pipeline also sets the stage for growth further.
Tech IPOs With Massive Profit Potential
In the past few years, many popular platforms and like Uber (NYSE:UBER) and Airbnb finally made their way to the public markets. But the biggest paydays came from lesser-known names.
For example, electric carmaker X Peng shot up +299.4% in just 2 months. Think of it this way…
If you had put $5,000 into XPEV at its IPO in September 2020, you could have cashed out with $19,970 in November.
With record amounts of cash flooding into IPOs and a record-setting stock market, this year’s lineup could be even more lucrative.
See Zacks Hottest Tech IPOs Now >>Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Regeneron Pharmaceuticals, Inc. (REGN): Free Stock Analysis Report
Sanofi (SNY): Free Stock Analysis Report
Roche Holding AG (OTC:RHHVF) (RHHBY): Free Stock Analysis Report
Bayer Aktiengesellschaft (BAYRY): Free Stock Analysis Report
To read this article on Zacks.com click here.
Zacks Investment Research

