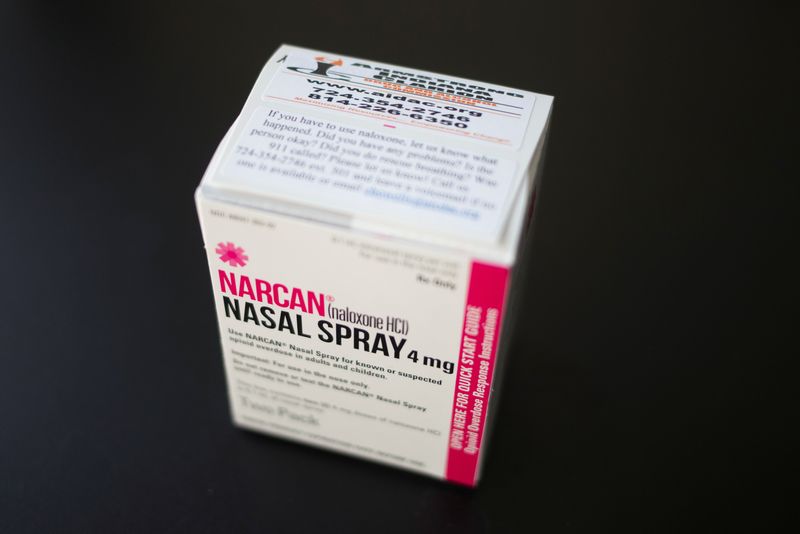
(Reuters) - Contract drugmaker Emergent Biosolutions (NYSE:EBS) said on Tuesday its over-the-counter nasal spray as a treatment for suspected opioid overdose would be reviewed on a priority basis by the U.S. health regulator.
Emergent is seeking the U.S. Food and Drug Administration's approval for a prescription-free sale of its nasal spray, Narcan, which is already cleared for the treatment of opioid overdose in the country.
The agency will make its decision by March 29 and its priority review status puts Narcan on track to become the first naloxone-based drug to be sold over the counter, Benchmark analyst Robert Wasserman said.
Emergent's announcement comes a few weeks after the FDA said naloxone might be safe and effective for over-the-counter use in some forms, potentially paving the way for its use federally.
There are legal barriers limiting access to naloxone in some states, and even in others the drug is not always available to those most at risk of an overdose.
The worsening opioid crisis has prompted U.S. President Joe Biden's administration to develop newer strategies, including the use of naloxone.
If approved, Narcan could face competition from generic versions of the drug, pressuring margins for Emergent, Cowen analyst Boris Peaker said.
Rival Opiant Pharmaceuticals (NASDAQ:OPNT)' drug nalmafene is also under the FDA's review and could pose additional risk to Narcan sales, as it provides better protection against an opioid overdose, Peaker added.
Government data estimates that there were more than 100,000 drug-related overdose deaths in the country during 2021, a near 15% increase from the year earlier.
The Maryland-based company said its application to the health regulator includes data that supports safe and effective use of Narcan based on usability and more than five years of post-marketing studies.