Shield Therapeutics PLC (LON:STXS) has announced positive top-line data from a Phase IIIb study (AEGIS-H2H) for Feraccru, a CHMP-approved oral formulation of iron positioned for the treatment of iron deficiency (ID) with or without anaemia. The data demonstrate that Feraccru is non-inferior to the market-leading IV iron (Ferinject) and has triggered a €2.5m payment from its marketing partner Norgine. Additional near-term revenue (royalties and milestones) can be expected as Norgine continues the rollout of Feraccru across Europe in 2020. In the US, we expect Feraccru approval in 2019 and that Shield will seek a marketing partner thereafter. We maintain our valuation of Shield at £178m or 153p/share.
Positive data will aid top-line growth for Shield
The AEGIS-H2H marketing study, comparing Feraccru (an oral iron treatment) to the market-leading IV iron (Ferinject), has reported promising top-line data; demonstrating that Feraccru is a non-inferior treatment option for IDA patients compared to Ferinject. We believe this data will increase clinical uptake and positively impact pricing negotiations and reimbursement. This will aid sales growth as partner Norgine continues to roll out Feraccru in Europe. We expect US Feraccru approval later this year (PDUFA date 27 July 2019). The AEGIS-H2H data strengthen Feraccru’s profile and will make the asset more attractive to a potential US marketing partner.
Feraccru demonstrates a non-inferiority complex
AEGIS-H2H was a multi-national Phase IIIb study in 242 inflammatory bowel disease (IBD) patients with iron deficiency anaemia (IDA). Shield has announced today that the primary endpoint of the study was met and after 12 weeks treatment on Feraccru, patients showed improvement in their haemoglobin levels within 9% (p = 0.022) to those receiving Ferinject (the current standard of care). The data demonstrate that Feraccru provides an alternative to IV iron in treating IDA. A more detailed analysis of the secondary endpoints can be expected at the company’s FY18 preliminary results (due in April 2019).
Valuation: £178m or 153p/share
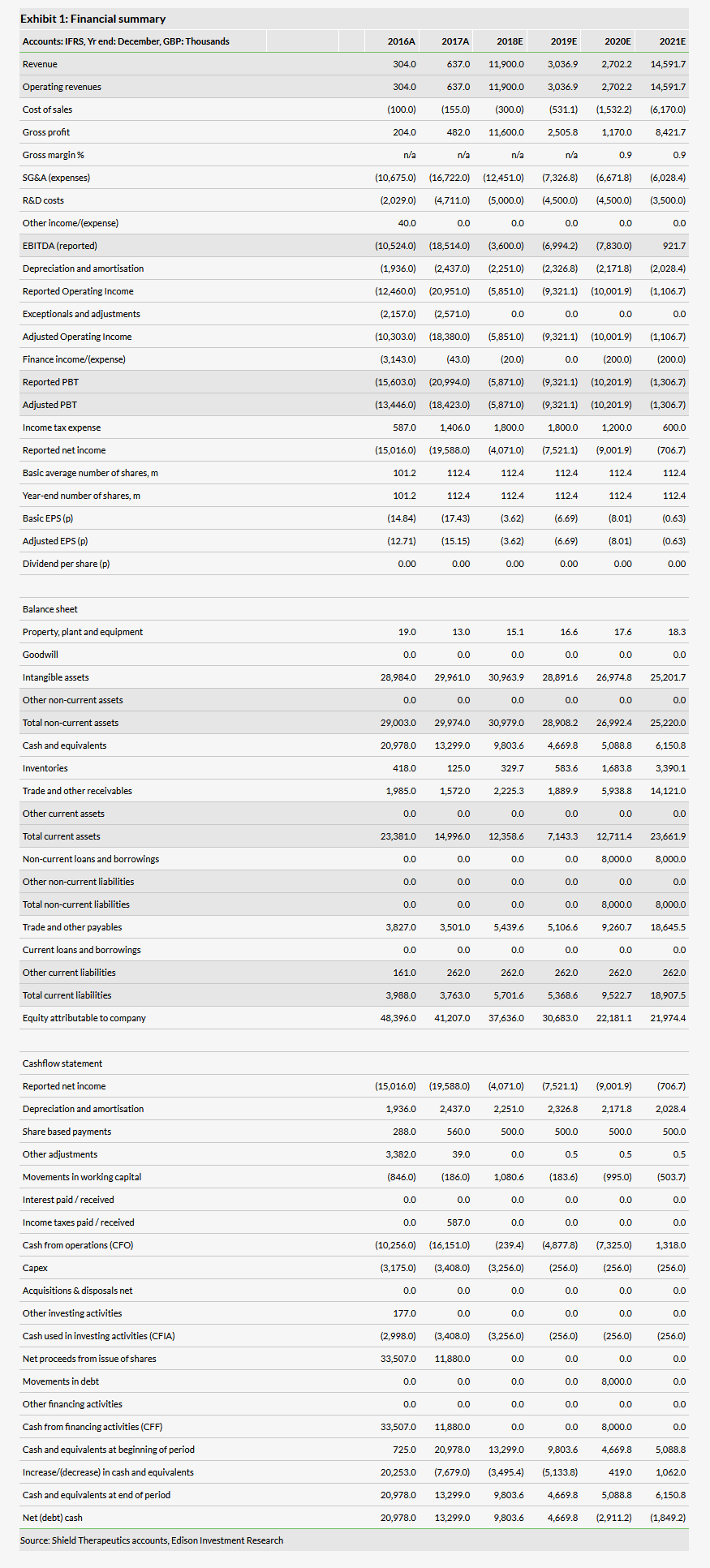
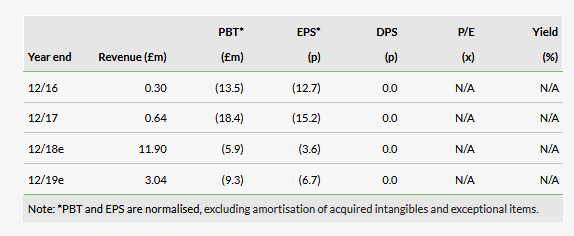
Our valuation of Shield remains unchanged at £178m or 153p/share. As detailed in our initiation note ‘Fortified for growth’, our valuation is based on a risk-adjusted NPV model of Feraccru for IDA in Europe and for CKD/IBD-related IDA in the US market. Our NPV calculation is based on Feraccru achieving 2029 peak sales of £334m in Europe (€133m) and the US ($251m), utilised a 10% discount rate and risk-adjusted the US opportunity accordingly (75% probability of success).
Business description
Shield Therapeutics is a commercial-stage pharmaceutical company. Its proprietary product, Feraccru, is approved by the EMA for iron deficiency and is undergoing review with the US FDA. Feraccru is currently marketed through partners Norgine, AOP Orphan and Ewopharma.