The following small cap company I find very interesting for a long term speculative buy. I actually have been able to ask the CEO of the company some questions that is part of my standard due diligence for me to make a determination whether a small cap company is worth writing about and/or investing in. Later on in this article, you will find a question and answer session between me and The CEO, Dr. Paul Ashton.
pSivida (PSDV) develops drug delivery products for treatment of back-of-the-eye diseases that are administered by implantation, injection, or insertion. The company's lead product candidate includes Iluvien, licensed to Alimera Sciences (ALIM). Iluvien is an drug that is inserted into the back of the eye that delivers fluocinolone acetonide (FA) for the treatment of diabetic macular edema (DME), a cause of vision loss. Alimera received its second CRL for the drug in November 2011, so Iluvien is not approved in the US. It is uncertain whether Alimera will pursue the clinical trials that the FDA would require for resubmission. However, Iluvien has been deemed "approvable" by the seven European countries where Alimera submitted its applications. Individual approvals are expected to be received in the coming months and will enable Alimera to commence marketing of Iluvien.
Alimera is also conducting Phase II clinical trials with Ilu for the treatment of wet and dry form of age-related macular degeneration, and retinal vein occlusion. These trials fall under the license agreement that pSivida has with Alimera.
pSivida's already-FDA approved products include Retisert for the treatment of posterior uveitis, an autoimmune condition characterized by inflammation of the posterior of the eye that can cause sudden or gradual vision loss; and Vitrasert for cytomegalovirus retinitis, a blinding eye disease that occurs in individuals with advanced AIDS. Both of these are licensed to Bausch & Lomb.
It is developing a Latanoprost product, an injectable, bioerodible drug delivery implant in Phase I/II dose-escalating study for the treatment of glaucoma and ocular hypertension; a Posterior Uveitis product candidate expected to start pivotal clinical trials shortly; its BioSilicon technology system, which is nano-structured porous silicon designed for use as a drug delivery platform and can deliver smaller molecules; and Tethadur, which utilizes BioSilicon to deliver biologic molecules, including peptides and proteins. It has strategic collaborations with Bausch & Lomb Incorporated; Alimera Sciences , and Pfizer (PFE)
PSDV's lead implant treatment is ILUVIEN, which is licensed to Alimera. The deal with Alimera in terms of outside The United States includes 20% net profits on a country by country basis and pSivida has full audit rights. If Alimera decides to partner out the European commercialization then PSDV is entitled to 33 percent of any upfront and or milestone payments and 20 percent of whatever Alimera gets in royalties.
I wrote an article on Alimera that covered the EU approval catalyst that was supposed to occur at the end of Q2, 2012. However, Alimera has now reported a delay in this process and does not expect approval to be gained to at least until later Q3. This certainly helped to throw off my projected short term price target opinion for Alimera.
It seems to me PSDV may have more to gain from the European approval. PSDV might get the bigger stock price percentage gain on the European approval news than Alimera may receive.
As referenced in my Alimera article, the FDA rejected ILUVIEN on its first attempt to gain approval, and issued a complete response letter (CRL) to Alimera.
After further research into PSDV, I have discovered that ILUVIEN is the not the product to be most optimistic about that PSDV is offering.
Tethadur could be the company's most valuable technology according to Dr. Paul Ashton, with its potential to deliver proteins peptides and other small molecules; particularly interesting for biosimilars and biobetters. Small molecule based procedures are more cost effective thancostlier biologic methods.
pSivida's Tethadur Technology is a platform drug delivery system that relies on nanostructuring to achieve optimal drug delivery. It can be used alone or in combination with pSivida's other technologies. Tethadur has:
- Ability to provide long-term delivery of anti-bodies and other proteins
- High efficiency/capacity of drug loading
- Controlled nanostructuring can vary nanosized pores to accommodate different molecule sizes
- Fully bioerodible over range of time periods
Unlike most polymer-based drug delivery systems, the manufacture of Tethadur does not require complex chemistry and the final product is pure silicon irrespective of the delivery characteristics imparted by the nanostructuring process.
Tethadur distinguishes itself from other delivery systems by its heat and radiation stability, simplifying the manufacturing and sterilization process.
pSivida is the kind of small cap bio I look for; grossly undervalued based on its technology platform and competent management.
The current management team eliminated $20 million in toxic debt inherited from the Australian management predecessors. I stress this over and over: The most important factor on success and/or failure of a small cap company is its management's ability to execute. Hype aside, good products mean nothing if management cannot execute correct business practices.
Money management, deal making, position leverage, communication with insurance companies, and other factors can and will determine a great deal of the company's success or lack thereof. I have given the prior example of Dendreon's (DNDN) Provenge, an effective treatment for prostrate cancer, yet insurance companies were not in an effective loop to fully understand the cost of the drug. Dendreon stock went on a wild ride all over the place, from $5 a share all the way up to $57+ a share, only to fall back below $8, mainly because of management's lack of proper communication. Most unfortunate, because Dendreon posesses great technology to make a real difference in the lives of many patients not only suffering from prostate cancer, but other forms of cancer as well. It does appear that Dendreon management has gotten its act together lately, but the damage was already done.
Should pSivida be selling for a price of around $2 a share currently? Let's take a look at its deep pipeline of treatments and find out.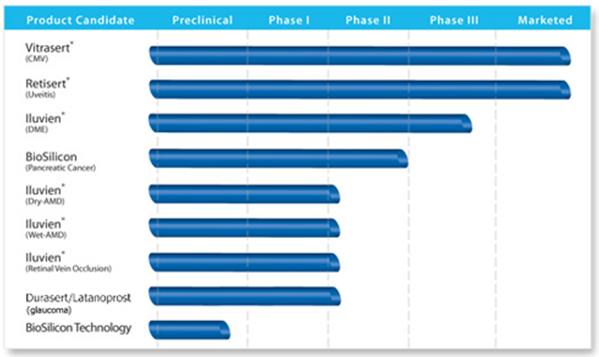
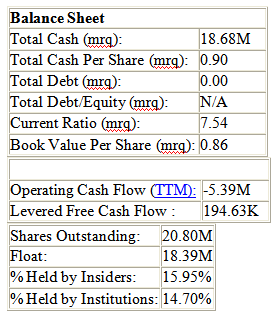
With a market cap of $40.56M, pSivida is one of the most undervalued companies I have ever seen. Nothwithstanding, the Alimera partnership, and its potential partnership with Pfizer values the company at least an $80M market cap in my strong opinion. The following sums up the Pfizer agreement:
In terms of the Pfizer agreement, at the moment Pfizer has no commitment to pSivida other than working with pSivida in terms of the phase I/II trials using the latanaprost in the insert. At the end of the Phase II trial, Pfizer has an option to pick up the product. If Pfizer exercises that option, then they would pay pSivida $20 million and Pfizer would take the product through Phase 3, and there would be performance milestone payments and a double digit royalty rate over 10%. Pfizer would then own the product. If Pfizer decides not to exercise its option, pSivida retains the right to develop and commercialize the glaucoma product on its own or with a partner. pSivida would also have royalty-free use of latanaprost (Pfizer's billion dollar drug that went off patent last year) as well.
Dr. Paul Ashton, CEO of pSivida, has a very solid resume of leadership as noted from his bio referenced from Forbes.com
Dr. Ashton was named pSivida's President and Chief Executive Officer in January 2009 and previously served as the company's Managing Director from January 2007 to January 2009 and Executive Director of Strategy from December 2005 to January 2007. From 1996 until its acquisition by pSivida in December 2005, Dr. Ashton was the President and Chief Executive Officer of Control Delivery Systems, Inc. (CDS), a drug delivery company that he co-founded in 1991 and that developed pSivida's Durasert technology system. Dr. Ashton previously was a joint faculty member in the Departments of Ophthalmology and Surgery at the University of Kentucky, served on the faculty of Tufts University and worked as a pharmaceutical scientist at Hoffman-La-Roche. Dr. Ashton's long history of leadership and strategic oversight of pSivida and CDS, his role in developing and extensive knowledge of pSivida's core technology platforms, products and product candidates, his scientific expertise including his PhD in pharmacology and strong knowledge of research and development uniquely position him to lead pSivida in the execution of its long-term strategy.
The following below is a question and answer session with Dr. Paul Ashton. I asked the standard questions I normally do when I am considering making an investment in a small cap growth company.
Scott: Hello Dr. Ashton, thank you for taking the time to answer my questions for Seeking Alpha readers.
Dr. Ashton: You are welcome Scott.
1. Where do you feel the true value in your company is where Investors should focus on?
2. I notice you have established a nice royalty base, do you plan to use this revenue to reinvest into the future of the company?
3. What do you find is the toughest challenge for a small company like yours to overcome?
4. While we know predicting the future is hard and many factors are uncertain, but where would you like to see the company in say, 5 years from now? What is the mission goal of the company in that time frame?
5. Do you feel your company has what it takes to eventually market your top line products on your own, is that your ultimate goal; using royalty revenue to eventually attempt to become a top line company?
Dr. Ashton's answers below in sequential order:
1. I think there is a lot of focus on Iluvien for Diabetic macular edema and appropriately so, however people may be overlooking the real upside.
1a. Our program in posterior uveitis (3rd largest cause of blindness in the US). In uveitis we are planning to develop an injectable implant delivering the steroid Fluocinolone acetonide. This is the exact same implant already successfully reviewed by the EU regulators for DME but rejected by the FDA. Why are we so confident? This insert delivers the exact same drug as Retisert (already approved by the FDA), the new inserts are injectable while Retisert is surgically inserted and the side effect profile of the new inserts is significantly better than that of Retisert. Also we can develop this product ourselves. Also based on our discussions with the FDA we believe we can proceed directly to phase iii clinical trials with these devices, referencing all the data from the DME application (a BIG time/money saver).
1b. The glaucoma program hasn't received any attention. This is another low risk, high reward opportunity. We are using a bio-erodible version of the Durasert technology (that underlies the Iluvien device) to deliver latanoprost, Pfizer's billion dollar glaucoma drug until the patent expired. So, it's an effective drug with a proven delivery system going after a very large market opportunity. It's in early clinical trials but as clinical data emerges I think it will get more attention.
1c. It's becoming increasingly apparent (see Alcon's (ACL) recent failure of a topical drug for geographic atrophy (a form of dry-AMD) Allergan's (AGN) failure of an oral for glaucoma,) that a drug delivery system is needed to treat back of the eye diseases. We are the only company with a proven back of the eye delivery technology
Dr. Ashton: Sorry that's a long answer, but we have a lot going on!
Scott: Understandable, I think as much detail as possible for investors to consider is a good thing.
2. Yes, historically we have used our royalties and cash from license fees etc to minimize our cash burn.
3. One of the biggest challenges we have is getting on the radar screen of investors. We have a couple of approved products earning royalties, a product pending marketing authorization in EU, we expect to have phase 3 ready product for uveitis very shortly and a glaucoma product already in the clinic. That seems pretty compelling to me, certainly better than many companies with a much higher market cap. I've been told many times "if your market cap was higher you would be worth more".
4. We are planning to evolve into a specialty pharma focused on ophthalmology. We plan to develop some of our own products (like for uveitis) and work with partners to develop others.
Dr. Ashton: We plan to out-license non-ophthalmic applications of our technologies.
5. We have the best technology and we are fortunate in that we are going after a new area (diseases of the back of the eye) where none of the big guys has a dominant position in the market. Also the back of the eye space can be addressed with a small sales force, there are only 1,500 retina specialists in the US. However this space now has the attention of a lot of the big players (Merck (MRK), Roche,(RHHBY.PK) Glaxo Smith Kline (GSK) etc) and there is a lot of consolidation, so I'd imagine that we'd likely be acquired before we get there. We are not planning this, but it seems to be the way things sometimes work.
Scott: Thank you Dr. Ashton for taking the time to answer my questions.
Dr.Ashton: You are welcome Scott.
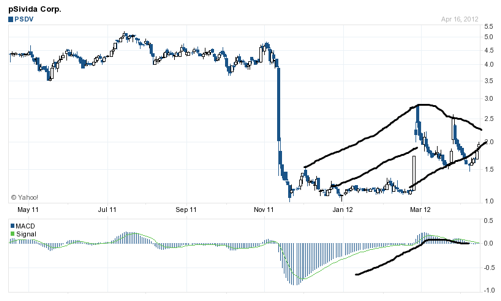
We can see from the chart above, the massive move down when the FDA issued a CRL to Alimera concerning ILUVIEN. Using crude black lines, I show where the stock channel connects up. There was a catalyst run-up concerning the EU approval of ILUVIEN, but that was delayed for another quarter and the stock sold off in March. Another gap up occured and has since been filled. Now it appears a tighter wedge is forming, indicating to me a move to near 2.50 is coming very soon. We see high lowers and lower highs representing the tighter wedge. The MACD has been somewhat flat, but the signal appears to be turning up. To me this seems stock holders are not willing to part with their shares at the current stock price level.
Key points in my opinion why pSivida has a legit shot to become a larger cap company over time:
- Very low cash burn rate; about two million a Quarter. The company tries to have agreements (evaluation agreements and license agreements) basically cover their costs on their programs.
- Nice capital structure; only about 20 million shares outstanding with an additional 5 million warrants and those are going down as groups of them expire. No debt. Cash that takes them with present programs through 2013.
- Opthalmology is a big area of interest as the population ages; take into account the success of Lucentis, which was developed by Genentech and is marketed in the United States by Genentech and elsewhere by Novartis (NVS). Also Regeneron's (REGN) eElea is having great success.
- Technologies that while obviously are for opthalmology now are adaptable for other parts of the body. (there is currently an evaluation agreement with the Hospital for Special Surgery in NYC for example, and their focus is orthopaedics). The uveitis program and the bioerodible version of the underlying technology of Iluvien for glaucoma with Pfizer (Pfizer is the second largest shareholder with about eight percent and have held the shares since 2007).
Since Pfizer already owns around 7% of the company's stock, pSivida is an acquisition target for them in my opinion. How strong of an acquisition target I really do not know.
Final opinion and price target opinions:
I see a short term move to near $2.50 setting up as the overall market appears to corrected itself, and the overall macro economic condition appears to have become more stable, at least in the short term. This should translate into more of a risk on trade appearing in the market again. I also see a catalyst run up occurring as Alimera gets closer to EU approval for ILUVIEN. I am not sure when this will begin or if it has already begun. A move to $5 a share by the end of the year is not out of the question. Where the price goes from there will be dependent on many factors including what Pfizer decides to do with its arrangement with pSivida. I like the CEO here as his team wiped off all the toxic financing the prior management left for them. Dr. Ashton is straight forward and to the point. Again, having top management in a small cap company is the number
one key in determining future success of the company. It is my opinion pSivida is a strong long term buy and hold for investors who wish to be patient and wait for large long term gains.
Disclosure: I have no positions in any stocks mentioned, and no plans to initiate any positions within the next 72 hours.
DISCLAIMER: This article is intended for informational and entertainment use only and should not be construed as professional investment advice, but rather my opinions as a writer only. Always do you own complete due diligence before buying and selling any stock.
