Pfizer, Inc. (NYSE:PFE) and partner Merck KGaA announced that the European Medicines Agency (EMA) has validated their type II variation application for PD-L1 inhibitor, Bavencio (avelumab) in combination with Inlyta for the treatment of patients with advanced renal cell carcinoma (RCC).
Per the regulatory agency, the application is complete and the EMA will now begin its review procedure.
The application was based on positive results from the phase III JAVELIN Renal 101 study, which showed that Bavencio and Inlyta significantly extended median progression-free survival (PFS) by more than five months compared with Sutent (sunitinib) as a first-line treatment for patients with advanced RCC.
We remind investors that the FDA has also accepted a supplemental Biologics License Application (sBLA) for Bavencio in combination with Inlyta for patients with advanced RCC for Priority Review, with a target action date in June 2019.
Inlyta is already approved in Europe for use in adult patients with advanced RCC after failure of prior treatment with Sutent or cytokine, while Bavencio is approved for the treatment of adults and pediatric patients aged 12 years or older with metastatic Merkel cell carcinoma (mMCC) and metastatic urothelial carcinoma (mUC).
Currently, avelumab is being developed for more than 15 types of tumors, including breast, gastric/gastro-esophageal junction, head and neck, Hodgkin’s lymphoma, melanoma, mesothelioma, Merkel cell carcinoma, non-small cell lung cancer, ovarian and urothelial carcinoma. These studies are part of the JAVELIN development program.
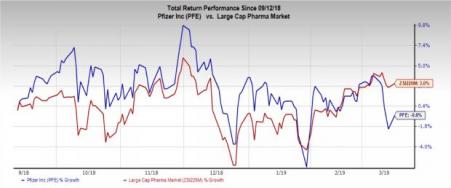
Pfizer’s stock has lost 0.8% in the past six months against the industry’s growth of 3%.
While the RCC market holds immense potential, competition is stiff. Last month, the FDA also granted priority review to Merck’s (NYSE:MRK) sBLA for Keytruda in combination with Inlyta for the first-line treatment of patients with advanced RCC based on the results of KEYNOTE-426, and has set a target action date of Jun 20, 2019.
Exelixis’ (NASDAQ:EXEL) Cabometyx is a dominant player in the market. The drug is approved for the treatment of patients with previously untreated advanced RCC and those with advanced RCC who have received prior anti-angiogenic therapy. The drug continues to gain market share with its broad label.
Competition has further stiffened up with the approval of Bristol-Myers’ (NYSE:BMY) Opdivo and Yervoy for the treatment of poor and intermediate risk first-line RCC.
Zacks Rank
Pfizer currently carries a Zacks Rank #4 (Sell).
You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Today's Best Stocks from Zacks
Would you like to see the updated picks from our best market-beating strategies? From 2017 through 2018, while the S&P 500 gained +15.8%, five of our screens returned +38.0%, +61.3%, +61.6%, +68.1%, and +98.3%.
This outperformance has not just been a recent phenomenon. From 2000 – 2018, while the S&P averaged +4.8% per year, our top strategies averaged up to +56.2% per year.
See their latest picks free >>
Pfizer Inc. (PFE): Free Stock Analysis Report
Merck & Co., Inc. (MRK): Free Stock Analysis Report
Bristol-Myers Squibb Company (BMY): Free Stock Analysis Report
Exelixis, Inc. (EXEL): Free Stock Analysis Report
Original post
Zacks Investment Research
