Pfizer (NYSE:PFE) and its Germany-based partner Merck KGaA announced that the Committee for Medicinal Products for Human Use (“CHMP”) of the European Medicines Agency (“EMA”) recommended approval of a label expansion application for PD-L1 inhibitor, Bavencio (avelumab), in first-line kidney cancer. A type II variation application was submitted by the companies to the EMA seeking approval of Bavencio in combination with Pfizer’s another cancer drug, Inlyta (axitinib) for the treatment of advanced renal cell carcinoma (“RCC”), the most common form of kidney cancer.
The CHMP opinion will be reviewed by the European Commission and a decision from the latter is expected in the fourth quarter of 2019.
Please note that the combination of Bavencio and Inlyta received approval in the United States for a similar indication in May. In January, a similar application had been submitted in Japan.
Bavencio is already approved for the treatment of adults and pediatric patients aged 12 years or older with metastatic Merkel cell carcinoma and metastatic urothelial carcinoma. Inlyta is also approved for use in adult patients with advanced RCC in second-line setting.
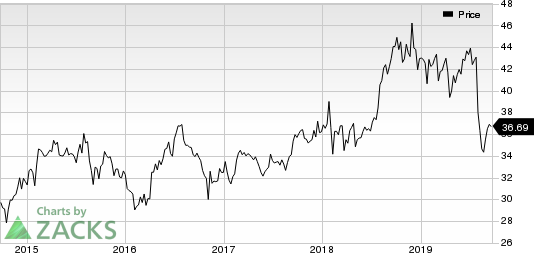
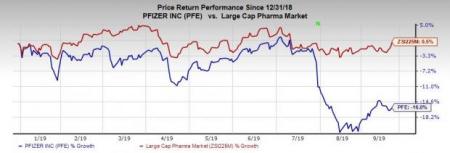
Pfizer’s stock has declined 16% so far this year against the industry’s increase of 0.5%.
The positive CHMP opinion was based on data from the phase III JAVELIN Renal 101 study, which evaluated the Bavencio/Inlyta combination against Pfizer’s older kidney cancer drug, Sutent (sunitinib). Data from the study, presented in the past, had shown that the combination regimen led to statistically significant improvement in progression-free survival and clinically meaningful improvement in objective response rate across all prognostic risk groups of patients.
Per the press release, 136,500 people in Europe were newly diagnosed with kidney cancer in 2018 and 54,700 people died due to the disease in the same year. Nearly 20% to 30% of the kidney cancer patients are diagnosed with advanced RCC. This represents a significant opportunity for the Bavencio + Inlyta combination.
While the RCC market holds immense potential, competition is stiff. Earlier this month, Merck’s (NYSE:MRK) Keytruda in combination with Inlyta was approved by the EC for the first-line treatment of patients with advanced RCC. In January, Bristol-Myers’ (NYSE:BMY) Opdivo and Yervoy was approved for the treatment of poor and intermediate risk first-line RCC. Exelixis’ (NASDAQ:EXEL) Cabometyx is an old player in the market.
Apart from RCC, Pfizer and its partner are developing Bavencio in several cancer indications under the JAVELIN program. Bavencio is being developed for more than 15 types of tumors, including breast, gastric/gastro-esophageal junction, head and neck, Hodgkin’s lymphoma, melanoma, mesothelioma, Merkel cell carcinoma, non-small cell lung cancer, ovarian and urothelial carcinoma.
Zacks Rank
Pfizer currently carries a Zacks Rank #4 (Sell).
You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Wall Street’s Next Amazon (NASDAQ:AMZN)
Zacks EVP Kevin Matras believes this familiar stock has only just begun its climb to become one of the greatest investments of all time. It’s a once-in-a-generation opportunity to invest in pure genius.
Bristol-Myers Squibb Company (BMY): Free Stock Analysis Report
Merck & Co., Inc. (MRK): Free Stock Analysis Report
Pfizer Inc. (PFE): Free Stock Analysis Report
Exelixis, Inc. (EXEL): Free Stock Analysis Report
Original post