Incyte Corporation (NASDAQ:INCY) reported strong results for the second quarter of 2019, wherein both earnings and sales comprehensively beat expectations. Shares are up in pre-market trading.
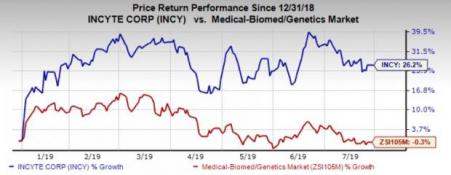
Shares of the company have gained 26.2% in the year so far against the industry’s 0.3% decline.
The company reported earnings of 75 cents per share, which easily surpassed the Zacks Consensus Estimate of 49 cents and 63 cents in the year-ago quarter.
Including milestones and contracts, revenues came in at $529.9 million, which increased 2% year over year and beat the Zacks Consensus Estimate of $501 million.
Quarter in Detail
Total product-related revenues came in at $433.9 million, up 18.7% from the year-ago quarter. Jakafi revenues came in at $409.5 million, increasing 18% from the year-ago quarter and beating the Zacks Consensus Estimate of $397 million. Net product revenues of Iclusig amounted to $24.4 million, up from $19.9 million in the year-ago quarter.
Product royalty revenues from Novartis AG (NYSE:NVS) for the commercialization of Jakafi in ex-U.S. markets grew 21% to $56.9 million. Olumiant’s product royalty revenues came in at $19.1 million.
R&D expenses were $262 million, down from $273 million in the year-ago quarter. SG&A expenses amounted to $93 million, down from $96 million in the prior-year quarter.
2019 Outlook Updated
The company expects Jakafi revenues of $1,610-$1,650 million in 2019 (previous guidance: $1,580-$1,650 million). Iclusig revenues are still expected to be $90-$100 million. R&D expenses are expected to be $1,020-$1,070 million. SG&A expenses are anticipated to be $420-$470 million.
Pipeline Update
Pipeline progress in the second quarter was impressive. In May 2019, the FDA approved a label expansion of Jakafi for the treatment of steroid-refractory acute graft-versus-host disease (GVHD) in adult and pediatric patients aged 12 years or older. This is the third indication, for which the drug has been approved in the United States.
The REACH2 and REACH3 trials, evaluating steroid-refractory acute and steroid-refractory chronic GVHD, respectively, are ongoing in collaboration with Novartis. Results are expected before the end of this year.
Results from the global phase III GRAVITAS-301 trial on itacitinib for the treatment of patients with newly-diagnosed acute GVHD are expected before the end of 2019 as well.
Incyte expects to submit the NDA for pemigatinib as a second-line treatment for patients with FGFR2 translocated cholangiocarcinoma in the second half of 2019. The company initiated a phase III trial for the first-line treatment of patients with cholangiocarcinoma in June.
Enrollment in the continuous dosing cohort of the phase II trial of pemigatinib in patients with bladder cancer is expected to complete by the end of 2019. A phase II study of pemigatinib in patients with driver-activations of FGFR is expected to open in the coming months.
The primary endpoint was met in the phase II trial of ruxolitinib cream in patients with vitiligo. The phase III development of ruxolitinib cream in patients with vitiligo is expected to begin by the end of the year.
Incyte has elected to no longer co-fund the development of baricitinib and in order to reallocate the capital to develop its pipeline. However, the company will continue to receive royalties on global net sales of Olumiant (baricitinib), per the terms of its agreement with partner Eli Lilly (NYSE:LLY) .
Our Take
Incyte’s performance in the second quarter was encouraging. The label expansion of Jakafi in acute GVHD should further boost sales. The company’s efforts to diversify its revenue base are impressive, but pipeline setbacks are a concern.
Zacks Rank & Stock to Consider
Incyte currently carries a Zacks Rank #3 (Hold).
A better-ranked stock in the biotech sector is Alexion Pharmaceuticals, Inc. (NASDAQ:ALXN) , which carries a Zacks Rank #2 (Buy). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Earnings estimates for Alexion have increased 35 cents for 2019 in the past seven days following strong second-quarter results.
Today's Best Stocks from Zacks
Would you like to see the updated picks from our best market-beating strategies? From 2017 through 2018, while the S&P 500 gained +15.8%, five of our screens returned +38.0%, +61.3%, +61.6%, +68.1%, and +98.3%.
This outperformance has not just been a recent phenomenon. From 2000 – 2018, while the S&P averaged +4.8% per year, our top strategies averaged up to +56.2% per year.
See their latest picks free >>
Eli Lilly and Company (LLY): Free Stock Analysis Report
Novartis AG (NVS): Free Stock Analysis Report
Alexion Pharmaceuticals, Inc. (ALXN): Free Stock Analysis Report
Incyte Corporation (INCY): Free Stock Analysis Report
Original post
Zacks Investment Research
