Shares of Agios Pharmaceuticals, Inc. (NASDAQ:AGIO) have soared 60.7% in a year’s time, outperforming the industry's increase of 25.1%.
Let’s analyze the factors that led to this rally.
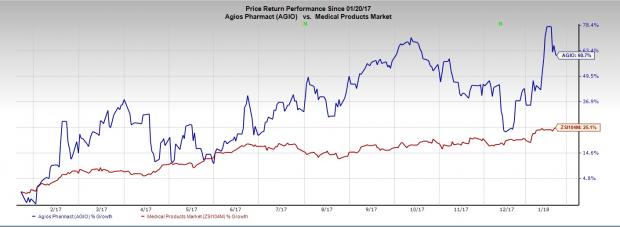
The FDA approval of the Agios’ only marketed drug Idhifa (enasidenib) last year and the company’s rapid progress on a robust pipeline in the past year accompanied with some study initiations and positive readouts have driven its share price consistently.
In August 2017, the FDA approved Idhifa for treating patients with relapsed or refractory acute myeloid leukemia (AML) with an isocitrate dehydrogenase-2 (IDH2) mutation. The approval offered a huge boost to the company given the immense commercial potential in the target market. Moreover, Idhifa provides a first-ever treatment, alternative to patients living with the aforementioned indication in the United States. Notably, Idhifa also enjoys an Orphan Drug status in the EU for treatment of AML.
Agios’ progress with the pipeline has been quite impressive. The company has some interesting candidates in its portfolio including an IDH1 mutant inhibitor AG-120 (ivosidenib) and a pan-IDH mutant inhibitor AG-881.
In June 2017, Agios presented positive data from the dose-escalation and expansion cohorts of the phase I study, evaluating single agent ivosidenib in mutant-positive cholangiocarcinoma at the ASCO.
Ivosidenib is also being evaluated in a phase I expansion cohort for treatment of patients with IDH1m R/R AML. Data from the study demonstrated durable responses which allowed the company to submit a new drug application (NDA) to the FDA for ivosidenib late in December.
During the same month, the company also announced positive results from a phase I study assessing ivosidenib in combination with standard induction chemotherapy or Celgene's (NASDAQ:CELG) Vidaza (Azacitadine) for treatment of newly diagnosed AML patients, not eligible for intensive chemotherapy.
Presently, a phase III AGILE study examining ivosidenib in combination with Vidaza is also underway for the given indication.
Apart from ivosidenib, Agios is conducting phase I trials on AG-881 for treatment of patients with advanced IDH1 or IDH2 mutant-positive solid tumors. Earlier in October, the company announced positive data from the study at the AACR-NCI-EORTC International Conference on Molecular Targets and Cancer Therapeutics.
Notably, the company also plans to initiate a perioperative window study to analyze ivosidenib and AG-881 in low grade glioma for investigating the effects on brain tumor tissue in the first half of 2018.
The company has another interesting candidate in its portfolio. Its lead rare genetic diseases candidate, AG-348, is under evaluation in a phase II study on adult, transfusion-independent patients with Pyruvate kinase (PK) deficiency. Agios reported positive data from the program in June 2017. The FDA granted a fast track designation to the candidate for the given indication. As the RGD market is still untapped, newer therapies in this area hold huge potential.
Successful development and commercialization of these investigational candidates should boost the company’s top line going forward considering the lucrative markets they are targeting.
Zacks Rank and Key Pics
Agios carries a Zacks Rank #3 (Hold). Two better-ranked stocks in the health care sector are Exelixis, Inc. (NASDAQ:EXEL) and Sucampo Pharmaceuticals, Inc. (NASDAQ:SCMP) . While Exelixis sports a Zacks Rank #1 (Strong Buy), Sucampo carries a Zacks Rank #2 (Buy). You can see the complete list of today’s Zacks #1 Rank stocks here.
Exelixis’ earnings per share estimates have been revised upward from 72 cents to 73 cents for 2018 over the last 60 days. The company delivered a positive earnings surprise in each of the trailing four quarters with an average beat of 572.92%. Share price of the company has soared 57% in a year’s time.
Sucampo’s earnings per share estimates have moved north from $1.15 to $1.19 for 2018 in the last 60 days. The company came up with a positive earnings surprise in three of the trailing four quarters with an average beat of 15.63%. Share price of the company has surged 57.9% in a year.
Will You Make a Fortune on the Shift to Electric Cars?
Here's another stock idea to consider. Much like petroleum 150 years ago, lithium power may soon shake the world, creating millionaires and reshaping geo-politics. Soon electric vehicles (EVs) may be cheaper than gas guzzlers. Some are already reaching 265 miles on a single charge.
With battery prices plummeting and charging stations set to multiply, one company stands out as the #1 stock to buy according to Zacks research.
It's not the one you think.
Agios Pharmaceuticals, Inc. (AGIO): Free Stock Analysis Report
Celgene Corporation (CELG): Free Stock Analysis Report
Exelixis, Inc. (EXEL): Free Stock Analysis Report
Sucampo Pharmaceuticals, Inc. (SCMP): Free Stock Analysis Report
Original post
Zacks Investment Research