Setting the stage for H214
GW Pharmaceuticals' (LONDON:GWP) pipeline should deliver important potentially transformative clinical data during H214. Major inflection points include Phase III cancer pain data (Q414) and US Phase III start in MS spasticity (H214/H115) for lead programme Sativex, as well as initial Epidiolex data in refractory childhood epilepsies from ongoing physician-led open-label studies. This disclosure, with periodic updates, will increase the body of clinical data related to Epidiolex use, and comes ahead of GW’s initiation of a placebo-controlled Phase II/III trial in Dravet Syndrome in H214.
Sativex: Sales growth; Phase III pain data upcoming
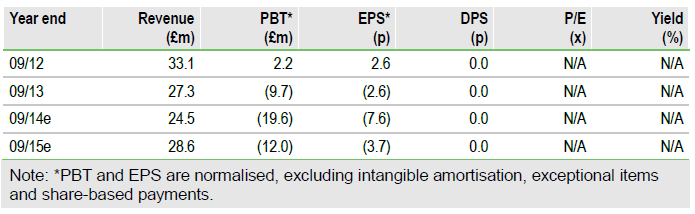
Europe in-market sales have grown 40% for H114 vs H113, driven mainly by sales into Germany and Italy. FDA Fast Track Status for cancer pain has been granted; initial Phase III data due by year end should support NDA filing in 2015. The Special Protocol Assessment process with the FDA for MS spasticity is ongoing.
Epidiolex: Initial data and GW trial start this year
Following IND authorisation for Dravet Syndrome (DS), a US Phase II/III study will start in H214, followed by a second Phase III in 2015. 60 children have been treated under physician-led expanded access treatment INDs (granted for c 300 children at 12 centres, up from 125 at five centres in January). Initial data from two centres, due mid-2014, will provide insight into Epidiolex’s profile, and expand clinical data. A pre-IND meeting for Lennox-Gastaut Syndrome is planned.
Other clinical programmes: Progressing steadily
In Phase I, GWP42006 was shown to be well-tolerated with no treatment-related adverse events; a pre-IND meeting for adult epilepsy, planned for H214, should define the development pathway. Data is expected in 2014 for GWP42003 (Phase IIa, ulcerative colitis) and THC/CBD (initial Phase Ib cohort data, glioma).
Valuation: Positive data could boost value to £1.1bn
Our risk-adjusted DCF of £792m or 366p/share (previously £692m) reflects new guidance and an increased Epidiolex success probability. Positive data from physician-led Epidiolex studies (mid-2014) and the start of the Phase II/III DS study (H214) would increase this to c £870m (c 400p). Progress in LGS would result in a valuation of c £1.1bn (c 500p), while positive Sativex Phase III cancer pain data (late 2014) would raise our DCF further to £1.1bn (518p/share). GW’s remaining R&D pipeline (epilepsy, ulcerative colitis and schizophrenia) offers pure upside.
To Read the Entire Report Please Click on the pdf File Below