Eli Lilly and Company (NYSE:LLY) announced positive results from a phase III RANGE study evaluating its oncology drug, Cyramza (ramucirumab), for expanded use in combination with docetaxel in patients with locally advanced or unresectable or metastatic urothelial carcinoma.
Note that Cyramza is already approved in the U.S. both as a single agent and when combined with another in the form of a second-line treatment for advanced or metastatic gastric cancer. In the case of metastatic NSCLC and metastaftic colorectal cancer, the drug is approved in combination with another agent, as a second-line treatment.
Shares of Lilly have underperformed the Zacks classified Large Cap Pharma industry so far this year. The stock has gained 8.2% compared to the broader industry’s 10.2%.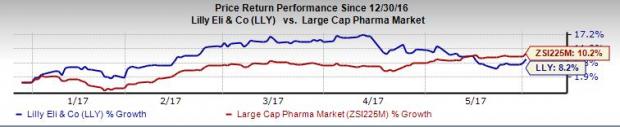
The RANGE study is designed to evaluate safety and efficacy of Cyramza in combination with docetaxel Vs placebo and docetaxel in patients with urothelial cancer. The study met its primary end-points and demonstrated an improved progression-free survival (PFS) in patients with the given indication. The study was globally conducted in 531 patients.
The secondary endpoints of the study include overall survival, objective response and disease control rates and duration of response. The company believes that results related to overall survival (OS) are likely to be required for global regulatory submissions. Lilly expects OS data by mid-2018.
We remind investors that Cyramza recorded sales of $171.2 million in the first quarter of 2017. Another drug, Alimta, is also a key product in the company’s oncology portfolio. After Alimta, Cyramza accounts for highest share in the company’s revenue from the oncology division. Cyramza’s contribution to revenues is crucial, considering the company’s top line is under pressure due to genericization of drugs like Alimta and Erbitux among others.
Per the company’s press release, bladder cancer accounts for majority of all urothelial carcinoma. Approximately 430,000 people worldwide are annually affected by bladder cancer, causing over 165,000 deaths.
In fact, in the U.S., bladder cancer is the sixth most common type of cancer. In 2017, around 79,000 new cases and nearly 17,000 deaths are estimated from bladder cancer. Hence approval of the drug for this indication will provide the company with access to a huge population base of patients affected by the disease.
The label expansion of Cyramza looks encouraging enough. Several studies on Cyramza are underway, evaluating it as a single agent and in combination with other anti-cancer therapies for treatment of multiple tumor types. It is being assessed under broad global development program that has enrolled over 10,000 patients across more than 70 trials worldwide. Label expansion of Cyramza should boost sales over the long term.
Zacks Rank & Key Picks
Lilly currently carries a Zacks Rank #3 (Hold). Better-ranked stocks in healthcare sector include VIVUS, Inc. (NASDAQ:VVUS) , Bayer (DE:BAYGN) AG (OTC:BAYRY) and Regeneron Pharmaceuticals, Inc. (NASDAQ:REGN) . While VIVUS sports a Zacks Rank #1 (Strong Buy), Bayer and Regeneron carry a Zacks Rank #2 (Buy). You can see the complete list of today’s Zacks #1 Rank stocks here.
VIVUS’s loss per share estimates tapered from 50 cents to 39 cents for 2017 in the last 30 days. The company posted positive earnings surprises in all four trailing quarters with an average beat of 233.69%.
Regeneron’s earnings per share estimates increased from $10.17 to $10.52 for 2017 and from $10.90 to $12.10, over last 30 days. The company posted positive earnings surprises in two of the four trailing quarters with average beat of 0.45%.
Bayer’s earnings per share estimates increased from $8.36 to $8.85 for 2017 and from $8.88 to $9.53 for 2018, over last 30 days. The company posted positive earnings surprises in three of four trailing quarters with average beat of 10.25%.
Will You Make a Fortune on the Shift to Electric Cars?
Here's another stock idea to consider. Much like petroleum 150 years ago, lithium power may soon shake the world, creating millionaires and reshaping geo-politics. Soon electric vehicles (EVs) may be cheaper than gas guzzlers. Some are already reaching 265 miles on a single charge.
With battery prices plummeting and charging stations set to multiply, one company stands out as the #1 stock to buy according to Zacks research.
It's not the one you think.
Eli Lilly and Company (LLY): Free Stock Analysis Report
Bayer AG (BAYRY): Free Stock Analysis Report
Regeneron Pharmaceuticals, Inc. (REGN): Free Stock Analysis Report
VIVUS, Inc. (VVUS): Free Stock Analysis Report
Original post
Zacks Investment Research