Elbit Med Tech (TA:EMTC)’ two portfolio investments continue to advance on multiple fronts. InSightec recently completed its compatibility project with Siemens MR scanners and its ExAblate Neuro units, which lends the opportunity to expand its presence in the global MR market. According to Gamida Cell, its cash balance should fund its operations through the top-line readout of its Phase III study of NiCord, which is expected in H120.
InSightec completes Siemens compatibility project
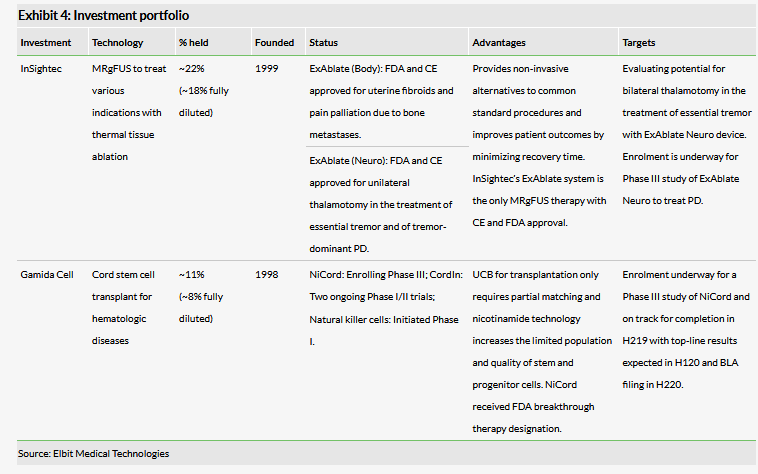
In December, InSightec (~22% owned by Elbit Medical, ~18% fully diluted) announced the receipt of FDA-expanded approval of ExAblate Neuro for the treatment of tremor-dominant Parkinson’s disease (PD). Notably, InSightec received the both FDA approval and CE mark for ExAblate Neuro compatibility with Siemens magnetic resonance (MR) scanners and we expect this to provide InSightec with the opportunity to expand its presence in the global MR market.
Gamida Cell looks to 2019 and 2020
Gamida Cell (~11% owned by Elbit Medical, ~8% fully diluted) has set clear milestones for the next few years. According to the company, its current cash position of $60.7m will provide a runway through March 2020, which is roughly in line with company expectations for delivering top-line NiCord data. Provided that these Phase III data are positive, Gamida Cell plans to submit a BLA filing for NiCord for the treatment of haematological malignancies in H220.
Elbit Imaging sells shares in Elbit Medical
Elbit Imaging recently entered into another share purchase agreement with Exigent Capital Group for the sale of between 1.6% and 25% of the ordinary shares of Elbit Medical for a price per share of NIS0.96 on or before 27 March 2019. Additionally, Exigent Capital may purchase up to 25% of shares through 13 May 2019 for a price per share of NIS1.02. As of 28 March 2019, 1.6% of Elbit Medical’s outstanding share capital was sold for NIS3.6m. Since August 2018, Elbit Imaging has sold roughly 27.6% of Elbit Medical’s outstanding share capital to Exigent Capital. Therefore, Elbit Imaging owns ~61.6% of Elbit Medical.
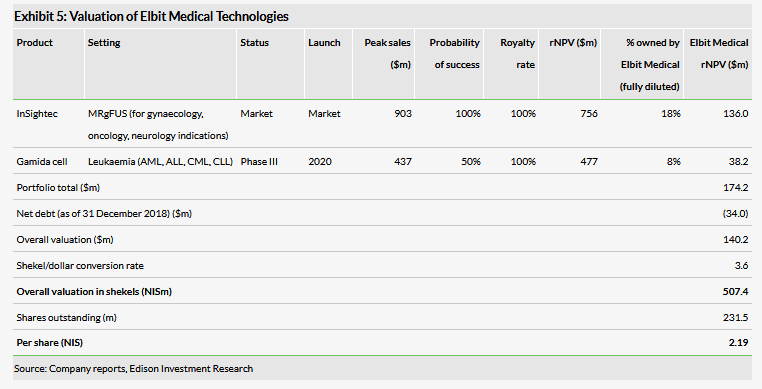
Valuation: NIS507.4m or NIS2.19 per share
We have increased our valuation to NIS507.4m or NIS2.19 per share, from NIS424m or NIS1.83 per share, primarily attributed to increasing the potential market share of ExAblate Neuro systems to include the opportunity of installing units on Siemens MR scanners. These changes were partially offset by the decrease of Elbit Medical’s stake (fully diluted) in Gamida Cell following its IPO.
Business description
Elbit Medical Technologies, a fully controlled subsidiary of Elbit Imaging, is an Israeli biomedical and healthcare technology group. Its portfolio of two companies is focused on medical devices and therapeutics: InSightec, which develops and markets the ExAblate platform for non-invasive thermal tissue ablation, and Gamida Cell, which is developing a universal bone-marrow transplant.
Expanded MR compatibility with Siemens
The ExAblate system comprises magnetic resonance imaging and high-intensity focused ultrasound (MRgFUS) to perform non-invasive thermal tissue ablation for a wide range of neurology, oncology and gynaecology clinical applications. By way of full clinical validation under the pre-market approval (PMA) route, the company has achieved FDA approval and CE markings for the ExAblate 2100 (Body) system for the treatment of symptomatic uterine fibroids and pain palliation caused by bone metastases, and for its ExAblate 4000 (Neuro) system for the treatment of medication-refractory ET (essential tremor). Moreover, the company has received CE markings for the treatment of prostate cancer, neuropathic pain and tremor-dominant PD. InSightec is investigating these devices further in clinical trials to achieve FDA approval and, on 18 December 2018, announced the FDA approved an expansion of ExAblate Neuro to include the treatment of patients with medication-refractory tremor-dominant PD. In February 2019, InSightec announced that Noridian posted positive local coverage determination for MRgFUS effective 1 April 2019 and that Medicare beneficiaries in 38 US states will have coverage for the treatment of ET using MRgFUS.
The clinical presence of the ExAblate platform is limited by the number of compatible MRI systems installed in hospital settings. We therefore believe the recent completion of the compatibility project for ExAblate Neuro with Siemens Magnetom Aera 1.5T and Skyra 3T clinical MR scanners in the US and in Europe could potentially double the accessible global MRI market for ExAblate Neuro. According to the Organisation for Economic Co-operation and Development, as of 2016 there were more than 5,400 MRI units active in US hospitals (roughly 16.67 per one million of the US population).1 This numeric excludes MRI units installed in outpatient imaging centres as we assume MRgFUS procedures will require the presence of a specialized surgeon. According to EvaluateMedTech, the diagnostic medical imaging market is led by Siemens Healthineers, GE Healthcare and Philips with 26%, 21% and 19% of the market share in 2016, respectively. Currently, the ExAblate Body systems are exclusively compatible with GE Healthcare’s 1.5 and 3.0 Tesla (NASDAQ:TSLA) MR scanners.
Disrupting the BBB using MRgFUS
On 30 January 2019, InSightec announced that the first patient completed temozolomide (TMZ) chemotherapy cycles in a clinical trial investigating the safety and efficacy MRgFUS for disrupting the blood brain barrier (BBB) in patients with glioblastoma. Gliomas are a collection of malignant tumours arising from glia or glial precursor cells within the central nervous system that form in the brain and spinal cord.2 Glioblastoma, also known as glioblastoma multiforme (GBM), is the most aggressive of the gliomas and affects less than 10 per 100,000 people in the US.2 Despite treatment (ie surgery to remove the tumour and adjuvant chemo- or radiation therapy) the disease is largely incurable and most patients with GBM have a median survival of about 14 to 15 months.3 Also, cytotoxic therapies such as chemotherapy do not effectively cross the BBB. The trial, which is being conducted at the University of Maryland Medical System in the US, is expected to enrol 15 patients with GBM and will analyse the number and severity of device and procedure related adverse events and investigate the feasibility of BBB disruption at the time of the procedure and 24 hours post-procedure.
Regarding the underlying business, InSightec recently reported its FY18 financials. Revenues, which are based on the sale of ExAblate systems and corresponding annual service contract costs and consumables, were $38.0m, up from $32.1m in FY17. R&D expenses totalled $28.4m for the year, which reflects ongoing clinical development.
Gamida Cell sets clear milestones for 2019–20
Gamida Cell’s 120-patient Phase III study of NiCord in patients with haematological malignancies is ongoing. NiCord, which is the company’s lead asset, expands umbilical cord blood (UCB) cell graft ex vivo and enriches the specific subpopulation of stem and progenitor cells to treat haematological malignancies such as leukaemia and lymphoma. Essentially, CD133+ cells selected from a single unit of UCB are cultured for 21 days in nicotinamide resulting in a c 100-fold expansion of dose stem and progenitor cells, which are then cryopreserved until they are transplanted into patients. This expansion is expected to provide a substantial advantage over a single UCB graft. The registrational trial is investigating the ability of NiCord to provide a graft with an ample number of cells that have fast and vigorous in vivo neutrophil- and platelet-producing potential to improve transplantation outcomes (as low cell dose is associated with delayed engraftment and poor outcomes). The primary endpoint for the trial is time to neutrophil engraftment following transplantation (on or before the 42nd day post-transplant) compared to a non-manipulated cord blood unit. The use of UCB for bone marrow transplantation (BMT) is limited by the minimal number of stem and progenitor cells. The NiCord process seeks to provide a more viable alternative to BMT in cancer patients, and only partial genetic matching is needed (ie a minimum requirement of four out of six HLA biomarkers). Enrolment is on track for completion in H219 with top-line data expected in H120. Provided that these Phase III data are positive, Gamida Cell plans to submit a BLA filing for NiCord for the treatment of haematological malignancies in H220.
The company is also investigating NiCord for the treatment of severe aplastic anaemia (SAA) in an ongoing Phase I/II study. Gamida Cell recently presented data on three patients included in the first cohort (Exhibit 1) with SAA and severe neutropenia who previously failed immunosuppressive therapy at the annual Transplantation & Cellular Therapy (TCT) Meetings of American Society for Blood and Marrow Transplantation and Center for International Blood and Marrow Transplant Research in Houston in February 2019.
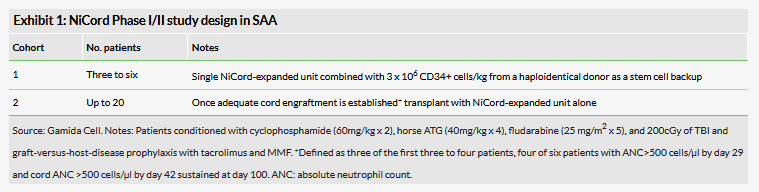
From 2017 to 2018, three SAA patients (age/gender: 22 male, 45 female, 22 female), who all previously failed immunosuppressive therapy, with a pre-transplant absolute neutrophil count (ANC) ≤ 500 cells/µl, successfully underwent a single 5/8 or 6/8 HLA-matched NiCord-expanded UCBT (UCB transplant) combined with haploidentical CD34+ cells from a haploidentical donor as a stem cell backup. For the three NiCord-expanded transplants, the median time to neutrophil and platelet recovery was six days (range six to seven) and 31 days (range 15–40), respectively. All three patients achieved cord engraftment (ANC>500 cells/µl) at a median of six days, which was sustained at day 100. Moreover, these patients were alive and free of graft-versus-host-disease at a median follow-up of 11 months (range four to 18 months). It is important to note these findings are based on only a small number of patients and significant variability between subjects was observed.
According to the company, patient inclusion in cohort one is complete, and it expects to proceed with cohort two to evaluate engraftment and transplantation outcomes with the NiCord-expanded unit alone (in other words, without a haploidentical donor) in 20 patients with SAA.
NAM-NK cells
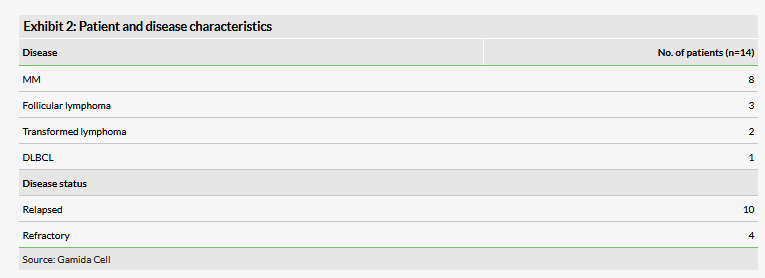
Gamida Cell is also developing donor-derived natural killer (NK) cells for blood and solid cancers. NK cells are a type of lymphocyte, or white blood cell, that play a central role in lysing infected or transformed cells and therefore offer an innovative approach to cancer treatment. The company previously initiated a 24-patient Phase I trial with the University of Minnesota evaluating the safety and activity of nicotinamide (NAM)-NK cells in patients with Non-Hodgkin’s lymphomas and multiple myeloma (MM). In February, preliminary data from 14 patients (Exhibit 2) were presented at the TCT annual meeting.
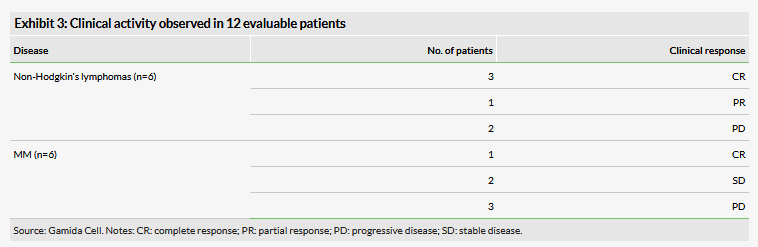
The objective of this Phase I study is to determine the maximum tolerated dose of NAM-NK. NAM-NK was generally well tolerated with no dose-limiting toxicities or infusion toxicity. However, several grade 3/4 hematologic toxicities were observed as well as low-grade non-haematological toxicities. The maximum target dose (MTD) of 2x108 cells/kg were achieved. Some clinical activity was observed in six patients with lymphomas and an additional six patients with MM who were evaluable for response (Exhibit 3). The trial remains ongoing and additional patients will be treated at the MTD to continue to evaluate safety and clinical activity. Gamida Cell expects to initiate a multi-centre Phase I/II study of NAM-NK in patients with blood cancers in 2020.
Also in February 2019, Gamida Cell announced an agreement with Editas Medicine, a genome-editing company, to evaluate the potential use of its CRISPR technology to edit Gamida’s NAM-NK cells. The two companies will engage in joint research focused on improving the tumour-killing properties of NAM-NK cells with CRISPR editing technology.
Gamida Cell (NASDAQ: GMDA, market capitalisation of $210m) recently reported its full-year financial results. The company reported a post-tax loss of $52.9m in FY18. R&D expenditure was $22.0m for FY18, which is up roughly 47% from FY17 ($15.0m), primarily attributed to the increase in clinical activities related to the advancement of the NiCord Phase III clinical programme in haematological malignancies and the initiation of the NAM-NK clinical programme. R&D spending is expected to increase substantially as the company advances its product candidates through clinical development. According to Gamida Cell, its cash position of $60.7m (including cash and equivalents, available-for-sale financial assets and short-term deposits) at 31 December 2018 will provide a runway through March 2020, which is roughly in line with company expectations for delivering top-line NiCord data. The company foresees the need for significant financing in the future.
Valuation
We have increased our valuation of Elbit Medical Technologies to NIS507.4m or NIS2.19 per share, from NIS424m or NIS1.83 per share. These changes are primarily driven by increasing the potential market share of ExAblate Neuro systems to include the possibility of installing units on Siemens MR scanners. Previously, both the ExAblate Neuro and Body systems were exclusively compatible with GE Healthcare’s MR scanners. The completion of the compatibility project with Siemens provides InSightec with the opportunity to expand its presence in the global MR market and increase the number of active ExAblate units. This change in overall valuation was also compounded by rolling forward our NPVs and the slightly lower net debt. These changes were partially offset by the decrease in strength of the US dollar (NIS3.62/US$), the decrease of Elbit Medical’s stake (fully diluted) in Gamida Cell following its October 2018 IPO, and the slight decrease of Elbit Medical’s stake (fully diluted) in InSightec.
Financials
Elbit Medical recently announced its full-year 2018 financial results. Its FY18 post-tax gain was $26.8m, mainly from its reduced stake in Gamida Cell following its IPO. General and admin costs for the year were $0.9m, which includes management fees, professional services and other related expenses. The company had cash of $5m (including cash and equivalents as well as restricted cash) at 31 December 2018 and $39.0m in debt. We outline historical financials in Exhibit 6. However, we are not providing forecasts at this time.