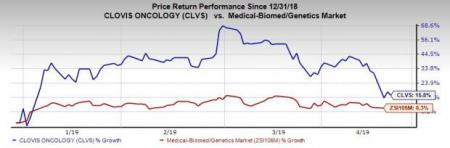
We issued an updated research report on Clovis Oncology, Inc. (NASDAQ:CLVS) on Apr 17. Shares of the company have increased 15.8% so far this year compared with the industry’s rise of 8.3%.
The commercial portfolio of Clovis consists of a single drug, Rubraca, which is approved as a maintenance treatment in recurrent ovarian cancer patients in second-line setting. The drug is also approved for treating BRCA mutated ovarian cancer in third or later-line setting. The company had in-licensed Rubraca from Pfizer (NYSE:PFE) in 2011.
PARP inhibitor, Rubraca’s sales increased sequentially in seven of the last eight reported quarters. The company’s commercialization efforts including the awareness programs aided sales. Net revenues, entirely from Rubraca, were approximately $95.4 million for full-year 2018, representing year-over-year growth of 71.8%.
Other PARP inhibitors approved for similar indications include Glaxo’s (NYSE:GSK) Zejula and Astrazeneca/Merck’s (NYSE:MRK) Lynparza. Sales of these two drugs have been significantly higher than Rubraca in 2018. With strong balance sheet of these pharma bigwigs, competition is expected to rise as they can spend more on commercialization initiatives.
However, Clovis remains focused on label expansion of Rubraca and launch of the drug in newer countries to boost the prospect of the drug. The company has collaborated with Merck and Bristol-Myers to develop Rubraca in combination with their PD-1/ PDL1 inhibitors, Keytruda and Opdivo, respectively. Apart from ovarian cancer, Rubraca is also being evaluated as monotherapy or in combination for treating several cancer indications including prostate, breast and gastroesophageal cancers, among others.
Clovis is looking to file a regulatory application for label expansion in prostate cancer later this year. Please note that the expansion of Rubraca’s label to include other cancer indications may also face stiff competition. In 2018, two PARP inhibitors were approved for treating breast cancer namely Lynparza and Pfizer’s Talzenna. However, there are no approved PARP inhibitors for prostate cancer yet.
In April 2019, Clovis discontinued phase II ATLAS study evaluating Rubraca monotherapy for metastatic bladder cancer as preliminary data suggested that it was unlikely to provide a meaningful clinical benefit to patients. Such setbacks hurt the prospects of the company.
Apart from Rubraca, the company is developing another candidate, lucitanib, for treating breast and lung cancer.
We will have to wait and see if the sales recovery that started in the fourth quarter continues. Moreover, the rise in mergers and acquisitions so far this year may lead to a buyout offer from any large pharma companies looking to boost their oncology portfolio.
Zacks Rank
Clovis currently has a Zacks Rank #4 (Sell).
You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Zacks' Top 10 Stocks for 2019
In addition to the stocks discussed above, would you like to know about our 10 finest buy-and-holds for the year?
Who wouldn't? Our annual Top 10s have beaten the market with amazing regularity. In 2018, while the market dropped -5.2%, the portfolio scored well into double-digits overall with individual stocks rising as high as +61.5%. And from 2012-2017, while the market boomed +126.3, Zacks' Top 10s reached an even more sensational +181.9%.
GlaxoSmithKline plc (GSK): Free Stock Analysis Report
Pfizer Inc. (PFE): Free Stock Analysis Report
Merck & Co., Inc. (MRK): Free Stock Analysis Report
Clovis Oncology, Inc. (CLVS): Free Stock Analysis Report
Original post
Zacks Investment Research