2013 will be a year in which ArQule ((ARQL)) and its partners are focused on progressing and/or completing clinical trials with tivantinib, but one which sees relatively few new clinical data read-outs. The speed of patient accrual in the recently opened METIV Phase III trial in hepatocellular carcinoma will, however, be an important signal. ArQule’s current up to 30 months target for recruitment could be very conservative. This will determine the timing of the interim analysis, which could come as early as late 2014. The start of a Phase I trial of ARQ 087 brings a new compound into clinical trials, while the future of ARQ 621 and ARQ 736 should soon be decided.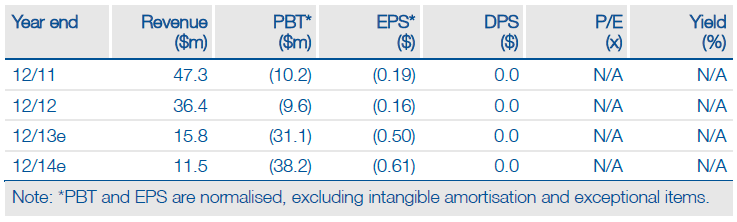
HCC Phase III trial underway
ArQule has now dosed the first patients in the METIV Phase III trial in HCC and aims to complete patient enrolment in two and half years, ie mid-2015, a timeline the management characterises as conservative. The trial has built in an efficacy and safety interim analysis, which could be potentially triggered in late 2014 if accrual and events accumulate fast. However, given announcements of several new Phase III trials in second-line HCC by competitors, ArQule may face more competition for patients. Nevertheless, we continue to expect tivantinib to reach the market in 2016.
Pipeline shuffle
ArQule has started a Phase I trial of ARQ 087, an orally bioavailable, multi-kinase inhibitor with pan-FGFR (fibroblast growth factor receptor) activity. At the same time, two other pipeline compounds, ARQ 621, an Eg5 inhibitor, and ARQ 736, a BRAF inhibitor, may be discontinued from development, pending final Phase I data and competitive landscape analysis. In addition, ArQule is conducting a Phase I study of ARQ 092, an AKT inhibitor for Daiichi Sankyo, which holds worldwide rights arising from a kinase inhibitor collaboration that ended in 2012.
Valuation: $300m or $4.30/per diluted share
We have revised our valuation to reflect a rebasing of the NPV to 2013 and to the company’s guided cash utilisation this year. We now value ArQule at $300m or $4.30 per share (fully diluted), vs our previous $4.65/share figure, based on our risk-adjusted DCF model. This includes tivantinib rNPV at $190m vs previously $165m, $25m for other assets and estimated 2013 year-end net cash of $85m. Our valuation for ArQule is significantly higher than its current market capitalisation, highlighting the potential for share price appreciation.
To Read the Entire Report Please Click on the pdf File Below.
- English (UK)
- English (India)
- English (Canada)
- English (Australia)
- English (South Africa)
- English (Philippines)
- English (Nigeria)
- Deutsch
- Español (España)
- Español (México)
- Français
- Italiano
- Nederlands
- Português (Portugal)
- Polski
- Português (Brasil)
- Русский
- Türkçe
- العربية
- Ελληνικά
- Svenska
- Suomi
- עברית
- 日本語
- 한국어
- 简体中文
- 繁體中文
- Bahasa Indonesia
- Bahasa Melayu
- ไทย
- Tiếng Việt
- हिंदी
ArQule: Post-Q4 Results Update
Published 03/19/2013, 08:36 AM
Updated 07/09/2023, 06:31 AM
ArQule: Post-Q4 Results Update
2013 will be a transitional year
Latest comments
Loading next article…
Install Our App
Risk Disclosure: Trading in financial instruments and/or cryptocurrencies involves high risks including the risk of losing some, or all, of your investment amount, and may not be suitable for all investors. Prices of cryptocurrencies are extremely volatile and may be affected by external factors such as financial, regulatory or political events. Trading on margin increases the financial risks.
Before deciding to trade in financial instrument or cryptocurrencies you should be fully informed of the risks and costs associated with trading the financial markets, carefully consider your investment objectives, level of experience, and risk appetite, and seek professional advice where needed.
Fusion Media would like to remind you that the data contained in this website is not necessarily real-time nor accurate. The data and prices on the website are not necessarily provided by any market or exchange, but may be provided by market makers, and so prices may not be accurate and may differ from the actual price at any given market, meaning prices are indicative and not appropriate for trading purposes. Fusion Media and any provider of the data contained in this website will not accept liability for any loss or damage as a result of your trading, or your reliance on the information contained within this website.
It is prohibited to use, store, reproduce, display, modify, transmit or distribute the data contained in this website without the explicit prior written permission of Fusion Media and/or the data provider. All intellectual property rights are reserved by the providers and/or the exchange providing the data contained in this website.
Fusion Media may be compensated by the advertisers that appear on the website, based on your interaction with the advertisements or advertisers.
Before deciding to trade in financial instrument or cryptocurrencies you should be fully informed of the risks and costs associated with trading the financial markets, carefully consider your investment objectives, level of experience, and risk appetite, and seek professional advice where needed.
Fusion Media would like to remind you that the data contained in this website is not necessarily real-time nor accurate. The data and prices on the website are not necessarily provided by any market or exchange, but may be provided by market makers, and so prices may not be accurate and may differ from the actual price at any given market, meaning prices are indicative and not appropriate for trading purposes. Fusion Media and any provider of the data contained in this website will not accept liability for any loss or damage as a result of your trading, or your reliance on the information contained within this website.
It is prohibited to use, store, reproduce, display, modify, transmit or distribute the data contained in this website without the explicit prior written permission of Fusion Media and/or the data provider. All intellectual property rights are reserved by the providers and/or the exchange providing the data contained in this website.
Fusion Media may be compensated by the advertisers that appear on the website, based on your interaction with the advertisements or advertisers.
© 2007-2024 - Fusion Media Limited. All Rights Reserved.