Ablynx’s technology platform received further validation from the promising Phase II study data for ALX-0061, an anti-IL6 Nanobody for rheumatoid arthritis (RA), as well as the formation of a new collaboration with Merck & Co. ALX-0061 seems to compare favourably with Roche’s Actemra, while offering the potential for a more convenient dosing schedule. The positive trial results could attract a partner for ALX-0061 and may even help with partnering of ATN-103 if the two products can be bundled together. We have increased our valuation by €20m to €521m.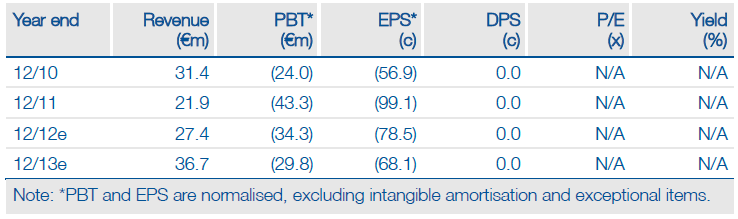
Promising Initial Data On ALX-0061
Initial data from a Phase II trial in RA with ALX-0061, an anti-IL-6R Nanobody, showed a promising evidence of efficacy, with encouraging improvements in the key markers of disease progression. Although a small trial, it suggests ALX-0061 is well tolerated. Furthermore, the Nanobody’s safety and efficacy profile would appear to compare favourably with Roche’s tocilizumab (Actemra), while potentially it could have a dose frequency advantage.
Aim To Partner ALX-0061 Could Help ATN-103
Ablynx needs an appropriate partner to progress the ALX-0061 programme. The strength of the data could attract some partners, but RA is very competitive. As well as Roche’s tocilizumab there are three other monoclonals targeting IL-6 or IL-6R in development. This competition led to UCB recently ceasing internal development of olokizumab (anti-IL-6). It is also possible that ALX-0061 and ATN-103 could be bundled together to provide an attractive package to a potential partner.
New Alliance With Merck & Co.
Ablynx has separately formed a new collaboration with Merck & Co to develop Nanobodies targeted towards a voltage-gated ion channel. The deal involves a €6.5m upfront and a €2m fee for research funding. A total of a further €448m in milestones could be payable as well as tiered royalties on eventual sales. There is an option for a second ion channel target with similar commercial terms.
Valuation: DCF Valuation Of €521m
We have increased our valuation by €20m to €521m after increasing the likelihood of ALX-0061 achieving peak sales of $900m by 20% to 30% and adding the potential milestone payments from the Merck & Co collaboration. The next significant catalyst could be the 24-week data on ALX-0061, an update on the potential licensing of ATN-103 or an announcement of another collaboration.
To Read the Entire Report Please Click on the pdf File Below.
- English (UK)
- English (India)
- English (Canada)
- English (Australia)
- English (South Africa)
- English (Philippines)
- English (Nigeria)
- Deutsch
- Español (España)
- Español (México)
- Français
- Italiano
- Nederlands
- Português (Portugal)
- Polski
- Português (Brasil)
- Русский
- Türkçe
- العربية
- Ελληνικά
- Svenska
- Suomi
- עברית
- 日本語
- 한국어
- 简体中文
- 繁體中文
- Bahasa Indonesia
- Bahasa Melayu
- ไทย
- Tiếng Việt
- हिंदी
Ablynx: Positive Clinical Trial Results
Published 10/12/2012, 10:25 AM
Updated 07/09/2023, 06:31 AM
Ablynx: Positive Clinical Trial Results
Study Data May Elicit A Partner
3rd party Ad. Not an offer or recommendation by Investing.com. See disclosure here or
remove ads
.
Latest comments
Install Our App
Risk Disclosure: Trading in financial instruments and/or cryptocurrencies involves high risks including the risk of losing some, or all, of your investment amount, and may not be suitable for all investors. Prices of cryptocurrencies are extremely volatile and may be affected by external factors such as financial, regulatory or political events. Trading on margin increases the financial risks.
Before deciding to trade in financial instrument or cryptocurrencies you should be fully informed of the risks and costs associated with trading the financial markets, carefully consider your investment objectives, level of experience, and risk appetite, and seek professional advice where needed.
Fusion Media would like to remind you that the data contained in this website is not necessarily real-time nor accurate. The data and prices on the website are not necessarily provided by any market or exchange, but may be provided by market makers, and so prices may not be accurate and may differ from the actual price at any given market, meaning prices are indicative and not appropriate for trading purposes. Fusion Media and any provider of the data contained in this website will not accept liability for any loss or damage as a result of your trading, or your reliance on the information contained within this website.
It is prohibited to use, store, reproduce, display, modify, transmit or distribute the data contained in this website without the explicit prior written permission of Fusion Media and/or the data provider. All intellectual property rights are reserved by the providers and/or the exchange providing the data contained in this website.
Fusion Media may be compensated by the advertisers that appear on the website, based on your interaction with the advertisements or advertisers.
Before deciding to trade in financial instrument or cryptocurrencies you should be fully informed of the risks and costs associated with trading the financial markets, carefully consider your investment objectives, level of experience, and risk appetite, and seek professional advice where needed.
Fusion Media would like to remind you that the data contained in this website is not necessarily real-time nor accurate. The data and prices on the website are not necessarily provided by any market or exchange, but may be provided by market makers, and so prices may not be accurate and may differ from the actual price at any given market, meaning prices are indicative and not appropriate for trading purposes. Fusion Media and any provider of the data contained in this website will not accept liability for any loss or damage as a result of your trading, or your reliance on the information contained within this website.
It is prohibited to use, store, reproduce, display, modify, transmit or distribute the data contained in this website without the explicit prior written permission of Fusion Media and/or the data provider. All intellectual property rights are reserved by the providers and/or the exchange providing the data contained in this website.
Fusion Media may be compensated by the advertisers that appear on the website, based on your interaction with the advertisements or advertisers.
© 2007-2024 - Fusion Media Limited. All Rights Reserved.