Three key regulatory events coupled with milestones from Novartis (NVS) are expected for Vectura (VEC.L) in H212: the EU and Japan approvals of NVA237 and the QVA149 European MAA filing. Data presentations at the European Respiratory Society (ERS) meeting provided further compelling data on the clinical profiles of these drugs. Detailed Phase III data for QVA149 showed superior lung function against all comparators, including its constituents (indacaterol and NVA237), current gold standard COPD therapies (Spiriva and Advair) and placebo. The closest LAMA/LABA competitor, GSK/Theravance’s UMEC/VI, fared less well, with dose ranging data that might raise FDA questions.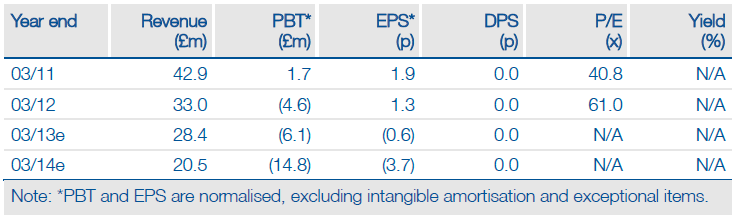
QVA149: Impressive against comparators
QVA149 should be the first marketed once-daily LAMA/LABA ex-US and Novartis views it as a “very important growth driver”. Phase III data from three trials confirmed its superior efficacy vs various comparators and a safety profile similar to placebo and potentially better than Advair. The latter point could be beneficial in supporting pricing and reimbursement and could also provide important differentiation vs Relvar/Breo.
NVA237: On track for 2012 launch
ERS presentations included pooled analysis of the GLOW1 and GLOW 2 trials. Safety and efficacy data support potential EU and Japan approval and launch by year end. NVA237 has shown improvement in trough FEV1 vs placebo of >100ml in five Phase III trials, including the QVA149 SHINE trial. Equivalence with Spiriva and better patient outcome measures should also support pricing and reimbursement.
Competitive dynamics: FDA dose focus may affect others
So far, QVA149 is the only LAMA/LABA for which the FDA has requested additional dosing data. Other programmes (UMEC/VI, tiotropium/olodaterol and LAS40464 ie aclidinium/formoterol) are all earlier stage; the latter two are still in Phase III. Global UMEC/VI filings are planned from end-2012, but Phase II UMEC dosing data at ERS raises concerns over dose selection for the completed UMEC/VI Phase III programme, which the FDA may want to explore further, delaying timelines.
Valuation: Risk-adjusted NPV of £416m (125p/share)
We maintain our £416m Vectura valuation, but expect to increase this after formal EU approval of NVA237 and EU filing of QVA149. Upside could come from regulatory and clinical progress, further regulatory clarity or further technology licences.
To Read the Entire Report Please Click on the pdf File Below.
- English (UK)
- English (India)
- English (Canada)
- English (Australia)
- English (South Africa)
- English (Philippines)
- English (Nigeria)
- Deutsch
- Español (España)
- Español (México)
- Français
- Italiano
- Nederlands
- Português (Portugal)
- Polski
- Português (Brasil)
- Русский
- Türkçe
- العربية
- Ελληνικά
- Svenska
- Suomi
- עברית
- 日本語
- 한국어
- 简体中文
- 繁體中文
- Bahasa Indonesia
- Bahasa Melayu
- ไทย
- Tiếng Việt
- हिंदी
Vectura: Three Key Regulatory Milestones Coming In H212
Published 09/13/2012, 07:55 AM
Updated 07/09/2023, 06:31 AM
Vectura: Three Key Regulatory Milestones Coming In H212
Compelling data at ERS
3rd party Ad. Not an offer or recommendation by Investing.com. See disclosure here or
remove ads
.
Latest comments
Install Our App
Risk Disclosure: Trading in financial instruments and/or cryptocurrencies involves high risks including the risk of losing some, or all, of your investment amount, and may not be suitable for all investors. Prices of cryptocurrencies are extremely volatile and may be affected by external factors such as financial, regulatory or political events. Trading on margin increases the financial risks.
Before deciding to trade in financial instrument or cryptocurrencies you should be fully informed of the risks and costs associated with trading the financial markets, carefully consider your investment objectives, level of experience, and risk appetite, and seek professional advice where needed.
Fusion Media would like to remind you that the data contained in this website is not necessarily real-time nor accurate. The data and prices on the website are not necessarily provided by any market or exchange, but may be provided by market makers, and so prices may not be accurate and may differ from the actual price at any given market, meaning prices are indicative and not appropriate for trading purposes. Fusion Media and any provider of the data contained in this website will not accept liability for any loss or damage as a result of your trading, or your reliance on the information contained within this website.
It is prohibited to use, store, reproduce, display, modify, transmit or distribute the data contained in this website without the explicit prior written permission of Fusion Media and/or the data provider. All intellectual property rights are reserved by the providers and/or the exchange providing the data contained in this website.
Fusion Media may be compensated by the advertisers that appear on the website, based on your interaction with the advertisements or advertisers.
Before deciding to trade in financial instrument or cryptocurrencies you should be fully informed of the risks and costs associated with trading the financial markets, carefully consider your investment objectives, level of experience, and risk appetite, and seek professional advice where needed.
Fusion Media would like to remind you that the data contained in this website is not necessarily real-time nor accurate. The data and prices on the website are not necessarily provided by any market or exchange, but may be provided by market makers, and so prices may not be accurate and may differ from the actual price at any given market, meaning prices are indicative and not appropriate for trading purposes. Fusion Media and any provider of the data contained in this website will not accept liability for any loss or damage as a result of your trading, or your reliance on the information contained within this website.
It is prohibited to use, store, reproduce, display, modify, transmit or distribute the data contained in this website without the explicit prior written permission of Fusion Media and/or the data provider. All intellectual property rights are reserved by the providers and/or the exchange providing the data contained in this website.
Fusion Media may be compensated by the advertisers that appear on the website, based on your interaction with the advertisements or advertisers.
© 2007-2024 - Fusion Media Limited. All Rights Reserved.