Teva Pharmaceutical Industries Ltd. (NYSE:TEVA) announced the launch of a generic version of Valeant Pharmaceuticals International, Inc.’s (NYSE:VRX) diabetes drug, Glumetza, in the U.S. The drug will be available in 500 mg and 1000 mg tablets.
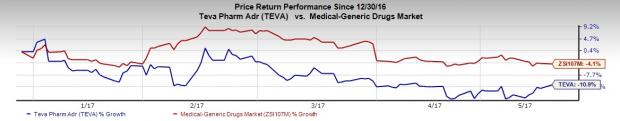
Teva’s shares have underperformed the Zacks classified Medical-Generics Drugs industry so far this year. Shares of the company lost 10.9%, while the industry registered a decrease of 4.1%.
As per IMS data, Glumetza recorded sales of $1.03 billion in one year ended Mar 2017. However, Lupin Pharmaceuticals Inc., the U.S. subsidiary of Indian pharma major – Lupin Limited – has been marketing a generic version of Glumetza since Feb 2016.
We remind investors that Teva is strengthening its generic business through acquisitions and developing generics of complex, high-quality products. With the acquisition of Allergan plc’s (NYSE:AGN) generic business – Actavis Generics – last year, Teva has the largest portfolio of FDA-approved generic products in the market.
Otsuka Agreement
In a separate press release, Teva declared entering into an agreement with Japan-based global healthcare company, Otsuka Pharmaceutical, to exclusively develop and commercialize Teva’s investigational drug candidate – fremanezumab – in Japan. The candidate is an anti-calcitonin gene-related peptide (CGRP) subcutaneous injection being developed for the prevention of episodic and chronic migraine.
Phase IIb global studies conducted by Teva showed that fremanezumab significantly reduced the number of monthly cumulative headache hours for chronic migraine and the number of migraine days for episodic migraine compared to baseline.
Otsuka will pay Teva an upfront payment of $50 million. The pharmaceutical company will further pay Teva on reaching various milestones of filing and regulatory approvals in Japan, and achieving specified revenue targets. Otsuka will be responsible in conducting and funding future clinical trials of fremanezumab in Japan.
Notably, Eli Lilly and Company (NYSE:LLY) had recently announced positive data from phase III studies of its anti-CGRP candidate, galcanezumab, for the same indications and is expected to submit a Biologics License Application (BLA) to the FDA in the second half of 2017.
Zacks Rank
Teva currently carries a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
5 Trades Could Profit "Big-League" from Trump Policies
If the stocks above spark your interest, wait until you look into companies primed to make substantial gains from Washington's changing course.
Today Zacks reveals 5 tickers that could benefit from new trends like streamlined drug approvals, tariffs, lower taxes, higher interest rates, and spending surges in defense and infrastructure. See these buy recommendations now >>
Eli Lilly and Company (LLY): Free Stock Analysis Report
Allergan PLC. (AGN): Free Stock Analysis Report
Valeant Pharmaceuticals International, Inc. (VRX): Free Stock Analysis Report
Teva Pharmaceutical Industries Limited (TEVA): Free Stock Analysis Report
Original post
