Sucampo’s investment case rests on its ability, with its partner Takeda, to protect and boost sales of constipation drug Amitiza (lubiprostone) in the US in the face of fresh competition from Ironwood/Forest’s Linzess (linaclotide). The entrance of Linzess in Q412 and a reinvigorated promotional effort behind Amitiza should help to grow the US market for the use of prescription drugs to treat constipation disorders (currently just 6-8%), while Amitiza’s proven track record provides a key differentiating factor.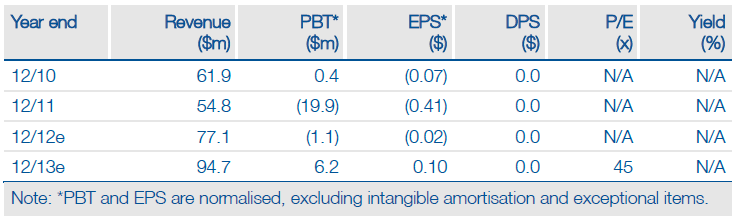
Proven Track Record
Over six million prescriptions have been filled in the US for Amitiza since it gained FDA approval in 2006. Until the recent approval for Linzess, Amitiza was the only available FDA-approved prescription drug for chronic idiopathic constipation (CIC) and irritable bowel syndrome with constipation (IBS-C). Given historical safety issues with using Rx drugs for the estimated ten million US patients seeking alternatives to dietary/lifestyle changes and OTC therapies, Amitiza’s established track record is a key selling point.
Promotional Piggy-Back And Line Extensions
Sucampo believes US Amitiza sales (FY11 $226m) are well below par, due to an inadequate promotional effort so far by Takeda, particularly in raising awareness of Amitiza among patients (the basis of its recently failed attempt to regain US rights). The launch of Linzess in Q412 could therefore benefit Amitiza, which holds further potential in opioid-induced constipation (FDA approval in H113), as well as near-term commercial opportunities in Japan (launch by Abbott Laboratories by end-2012) and Europe (UK approval for CIC in Sep 2012 and potential to secure new partners).
Rescula Opportunity
Sucampo is planning to launch Rescula (unoprostone) in the US by the end of 2012 as a treatment to reduce intra-ocular pressure (IOP) in glaucoma patients, an important milestone in building Sucampo’s commercial abilities in a burgeoning ophthalmology sector and expanding the use of its prostone-based products.
Valuation: $304m With Upside Potential
We value Sucampo at $304m, or $7.25 per share, based on a sum-of-the-parts DCF valuation. This assumes Amitiza’s current US sales run rate is maintained and includes risk-adjusted new opportunities in OIC in the US and Japan for CC, as well as a successful launch for Rescula in the US. Our base-case valuation represents clear upside to Sucampo’s market capitalisation of $193m and $4.60 share price.
To Read the Entire Report Please Click on the pdf File Below.
- English (UK)
- English (India)
- English (Canada)
- English (Australia)
- English (South Africa)
- English (Philippines)
- English (Nigeria)
- Deutsch
- Español (España)
- Español (México)
- Français
- Italiano
- Nederlands
- Português (Portugal)
- Polski
- Português (Brasil)
- Русский
- Türkçe
- العربية
- Ελληνικά
- Svenska
- Suomi
- עברית
- 日本語
- 한국어
- 简体中文
- 繁體中文
- Bahasa Indonesia
- Bahasa Melayu
- ไทย
- Tiếng Việt
- हिंदी
Sucampo Pharmaceuticals Initiation Of Coverage
Published 09/28/2012, 07:58 AM
Updated 07/09/2023, 06:31 AM
Sucampo Pharmaceuticals Initiation Of Coverage
Prostone Pioneer
Latest comments
Loading next article…
Install Our App
Risk Disclosure: Trading in financial instruments and/or cryptocurrencies involves high risks including the risk of losing some, or all, of your investment amount, and may not be suitable for all investors. Prices of cryptocurrencies are extremely volatile and may be affected by external factors such as financial, regulatory or political events. Trading on margin increases the financial risks.
Before deciding to trade in financial instrument or cryptocurrencies you should be fully informed of the risks and costs associated with trading the financial markets, carefully consider your investment objectives, level of experience, and risk appetite, and seek professional advice where needed.
Fusion Media would like to remind you that the data contained in this website is not necessarily real-time nor accurate. The data and prices on the website are not necessarily provided by any market or exchange, but may be provided by market makers, and so prices may not be accurate and may differ from the actual price at any given market, meaning prices are indicative and not appropriate for trading purposes. Fusion Media and any provider of the data contained in this website will not accept liability for any loss or damage as a result of your trading, or your reliance on the information contained within this website.
It is prohibited to use, store, reproduce, display, modify, transmit or distribute the data contained in this website without the explicit prior written permission of Fusion Media and/or the data provider. All intellectual property rights are reserved by the providers and/or the exchange providing the data contained in this website.
Fusion Media may be compensated by the advertisers that appear on the website, based on your interaction with the advertisements or advertisers.
Before deciding to trade in financial instrument or cryptocurrencies you should be fully informed of the risks and costs associated with trading the financial markets, carefully consider your investment objectives, level of experience, and risk appetite, and seek professional advice where needed.
Fusion Media would like to remind you that the data contained in this website is not necessarily real-time nor accurate. The data and prices on the website are not necessarily provided by any market or exchange, but may be provided by market makers, and so prices may not be accurate and may differ from the actual price at any given market, meaning prices are indicative and not appropriate for trading purposes. Fusion Media and any provider of the data contained in this website will not accept liability for any loss or damage as a result of your trading, or your reliance on the information contained within this website.
It is prohibited to use, store, reproduce, display, modify, transmit or distribute the data contained in this website without the explicit prior written permission of Fusion Media and/or the data provider. All intellectual property rights are reserved by the providers and/or the exchange providing the data contained in this website.
Fusion Media may be compensated by the advertisers that appear on the website, based on your interaction with the advertisements or advertisers.
© 2007-2024 - Fusion Media Limited. All Rights Reserved.