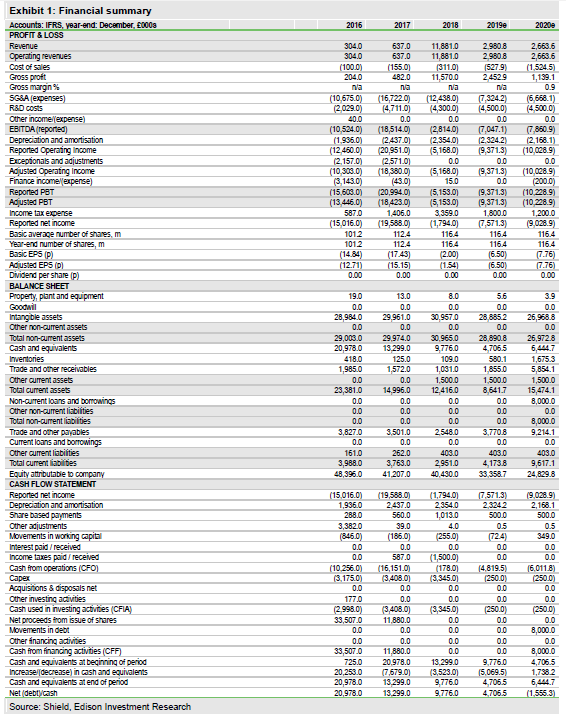
Shield Therapeutics PLC (LON:STXS) reported FY18 results in line with our expectations. Total revenue of £11.9m included the £11.0m upfront payment from European partner Norgine. Operating expenses were substantially reduced as Shield moved from a direct selling model to out-licensing deals for Feraccru commercialisation. Norgine will roll out Feraccru across additional European countries in 2020, subject to country-by-country reimbursement negotiations. In the US, we expect Feraccru approval in 2019 and that Shield will seek a marketing partner thereafter. The positive AEGIS-H2H data (non-inferiority to IV iron) announced recently should strengthen Feraccru’s appeal. We value Shield at £177m (from £178m).
Business description
Shield Therapeutics is a commercial-stage pharmaceutical company. Its proprietary product, Feraccru, is approved by the EMA for iron deficiency and is undergoing a review with the US FDA. Feraccru is currently marketed through partners Norgine, AOP Orphan and Ewopharma.
Next events
Feraccru US PDUFA date: 27 July 2019
Feraccru launches in additional European countries: 2020
2019 US approval and marketing partner
Key inflections in 2019–20 include potential regulatory approval in the US (and a partnering deal), sales growth across Europe and the US, and potential China out-licensing of Feraccru (discussions with Chinese companies are underway). Partnering strategies enhance economic returns and de-risk the investment case. The AEGIS-H2H marketing study, comparing Feraccru (an oral iron treatment) to the market-leading IV iron (Ferinject), has reported promising top-line data, demonstrating that Feraccru is a non-inferior treatment option for patients with iron deficiency anaemia compared to Ferinject. We believe these data will increase clinical uptake, and positively affect pricing negotiations and reimbursement.
Financials: Cash runway into 2020
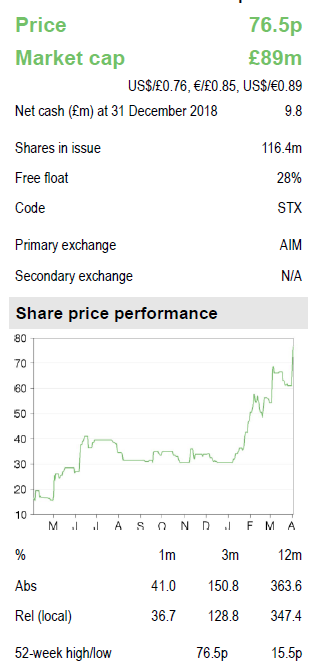
Shield reported a net loss of £1.8m in FY18 (FY17: £19.6m), benefiting from the £11m upfront license payment from Norgine and a significant reduction in operating expenses following the restructuring of the company in 2018. The year-end cash position of £9.8m implies a cash runway into 2020. We forecast a 2019 cash burn of £5.1m and sustainable profitability from 2022. The shield is dependent in the near term on royalty and milestone income from partners. A US partnering deal in 2019 should enable an upfront licensing payment to extend cash runway further.
Valuation: £177m or 152p/share
Our valuation of Shield is at £177.4m or 152p/share (153p/share before). We have rolled forward our model and updated FX rates. As detailed in our initiation note, Fortified for growth, our valuation is based on a risk-adjusted NPV model of Feraccru for IDA in Europe and for CKD/IBD-related IDA in the US market. Our NPV calculation is based on Feraccru achieving 2029 peak sales of €133m in Europe and $251m in the US, utilizes a 10% discount rate and risk-adjusts the US opportunity accordingly (75% probability of success).