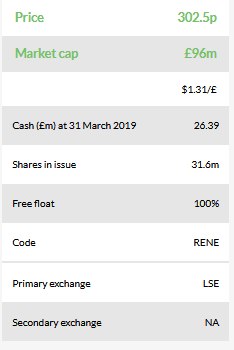
ReNeuron (LON:RQE)’s year-end update summarised its recent clinical progress in the human retinal progenitor cell (hRPC) programme in retinitis pigmentosa (RP), which has become the focus for investors, and a slight delay to its CTX programme for stroke disability. The announced £26.4m cash position was slightly higher than our prior YE19 forecast of £25.7m and will help ReNeuron’s position in any further partnering discussions.
hRPC moves to centre stage
The recent early but striking data on ReNeuron's hRPC product for treating RP has become the focus of investor attention. This is because the data reported so far are good, the study has a placebo-controlled element (with one of the patient’s eyes untreated) and the results for all 12 of the patients in the Phase IIa study are expected to be reported in H219. We have not changed the timelines for the first regulatory approval for the hRPC product, which we had estimated to be in 2023, but the possibility exists that this timeline could accelerate if the data continue to prove compelling. In a similar way, the clinical trial results to date have raised the profile of this programme and, bearing in mind the cash-rich, innovative product-poor status of many big pharmaceutical and biotechnology companies, we would not be surprised to see another licensing transaction for the hRPC product.
Minor delay on CTX but robust cash position
In its year-end update, ReNeuron announced a minor delay to its lead CTX programme for stroke disability with results from the PISCES III study now expected in H220, rather than H1. We have not changed our launch timing of 2023 for the CTX product. We had included relatively conservative assumptions in our model that could absorb a six-month delay and, in any event, approval is expected to require two pivotal studies. ReNeuron’s update also noted the increase in business development activities and its £26.4m cash position – sufficient to support operations for at least the next year. This should enable ReNeuron to maintain a robust stance in any business development discussions that could include hRPC, CTX products, or the exosome drug delivery platform.
Valuation: Minor changes prior to FY19 results in July
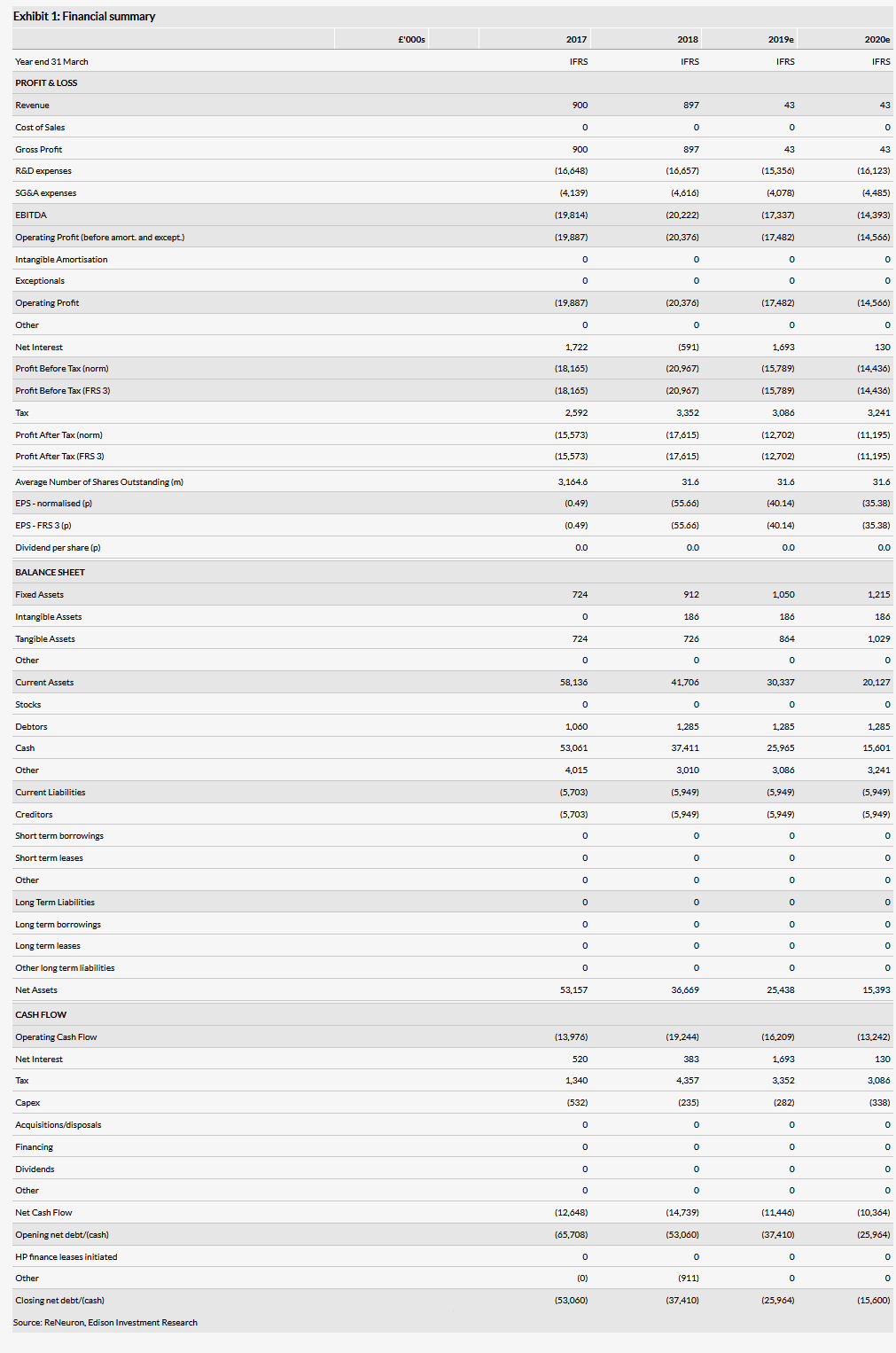
We have moved the recognition of the Fosun upfront payment, which we had previously estimated in FY19, to FY20. We have also trimmed FY19 R&D spend by c £7m to take into account the slower start of the CTX study. Our valuation has changed slightly due to these modifications and the revised FY19 cash balance from £199m or 630p per share to £193m or 610p per share.
Business description
ReNeuron is a UK biotech company developing allogeneic cell therapies. The first pivotal Phase IIb trial for CTX neural stem cells for chronic stroke disability is underway. Human retinal progenitor cells are also being studied for RP (in Phase I/IIa).