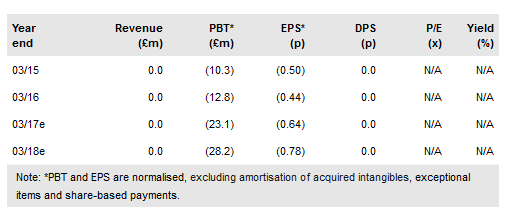
Reneuron Group (LON:RQE) has presented positive Phase II trial data for its CTX cells in chronic stroke patients. Reported data included 15 out of 21 patients having a clinically relevant and sustained response on at least one efficacy measure. This confirms the potential for long-term benefits from treatment with its CTX neural stem cells and has led to the decision to progress to a pivotal controlled clinical study in 2017. Beyond CTX, we expect safety and efficacy data from its retinitis pigmentosa (RP) trial in 2017. Our rNPV has increased to £278m (vs £249m) as we have increased the probability of the stroke programme’s success to 25% (from 20%) and updated cash.
Positive Phase II (PISCES II) stroke trial results
PISCES II was a single arm, open-label study in patients that had stable paresis (partial paralysis) of an arm four to 12 weeks following an ischaemic stroke. The focus was on a sub-group of stroke patients with no useful upper limb function at four to eight weeks post stroke. The primary outcome measure was a minimum 2-point improvement in the ARAT (Action Research Arm Test), a grasping and lifting test, at three months post treatment. While this was not met within the pre-set timeframe of three months, three of the 21 patients achieved it at various time points within 12 months. Equally, other endpoints of the study were met, which led to 15 out of 21 patients achieving a clinical relevant and sustained response on at least one efficacy measure. Importantly, CTX was shown to be well tolerated with side effects only relating to the surgical procedure. ReNeuron now intends to apply to the US and EU regulators for permission to commence a randomised, placebo-controlled, pivotal clinical trial in patients in 2017, with potential read-out in 2019.
To read the entire report Please click on the pdf File Below