Regeneron Pharmaceuticals, Inc. (NASDAQ:REGN) announced that the Ministry of Health, Labor and Welfare (MHLW) in Japan has granted marketing and manufacturing authorization for the label expansion of IL-4/IL-13 pathway-blocking antibody, Dupixent (dupilumab). The drug is approved for the treatment of atopic dermatitis (AD) in adults not adequately controlled with existing therapies.
Regeneron develops Dupixent along with Sanofi (NYSE:SNY) under a global collaboration agreement, and it will be commercialized in Japan by Sanofi.
Dupixent injection is presently marketed in the United States for the treatment of adults with moderate-to-severe AD. It is the first and only biologic medicine approved for the treatment of adults suffering from AD. It was approved in the United States in March 2017. It was approved for AD in the EU in September 2017.
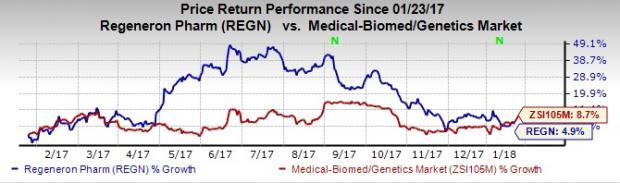
Shares of Regeneron have rallied 4.9% compared with the industry’s gain of 8.7% over a year.
Apart from AD, Dupixent is also being evaluated for eosinophilic esophagitis and other inflammatory indications including asthma and nasal polyposis in late stage studies. In September 2017, Sanofi and Regeneron announced that Dupixent met its two primary endpoints in a phase III asthma study. Data from the LIBERTY ASTHMA QUEST study showed that Dupixent, when added to standard therapies, reduced severe asthma attacks and improved lung function.
Earlier this month, Regeneron and Sanofi announced that they will expand their investment for the label expansion of Dupixent, in Type II allergic diseases. The increased investment in the label expansion of dupilumab development program will enable the companies to speed up the planned new studies in chronic obstructive pulmonary disease, peanut allergy and grass allergy as well as in patients who have multiple allergic conditions.
Zacks Rank & Stocks to Consider
Regeneron has a Zacks Rank #3 (Hold).
Some better-ranked stocks from the health care space are Exelixis, Inc. (NASDAQ:EXEL) and XOMA Corporation (NASDAQ:XOMA) , both carrying a Zacks Rank #1 (Strong Buy). You can see the complete list of today’s Zacks #1 Rank stocks here.
Exelixis’ earnings per share estimates have moved up from 72 cents to 73 cents for 2018 in the last 60 days. The company delivered a positive earnings surprise in all the last four quarters, with an average beat of 572.92%. Share price of the company surged 53.1% in the past one year.
XOMA’s loss per share estimates have narrowed from 99 cents to 42 cents for 2018 in the last 60 days. The company pulled off a positive earnings surprise in one of the last four quarters, with an average beat of 47.92%. Share price of the company skyrocketed 656.3% in the past one year.
Wall Street’s Next Amazon (NASDAQ:AMZN)
Zacks EVP Kevin Matras believes this familiar stock has only just begun its climb to become one of the greatest investments of all time. It’s a once-in-a-generation opportunity to invest in pure genius.
Sanofi (SNY): Free Stock Analysis Report
Regeneron Pharmaceuticals, Inc. (REGN): Free Stock Analysis Report
XOMA Corporation (XOMA): Free Stock Analysis Report
Exelixis, Inc. (EXEL): Free Stock Analysis Report
Original post
Zacks Investment Research