In May 2019, Redhill Biopharma Ltd (NASDAQ:RDHL) submitted an NDA for TALICIA for the treatment of H. pylori infection. If all goes according to plan, the drug could be launched by end-2019. This follows positive top-line results from the TALICIA confirmatory Phase III study in first-line treatment of H. pylori infection regardless of ulcer status announced late last year. RedHill plans to use its existing commercial platform to market TALICIA to healthcare practitioners. Other projects in the R&D pipeline are also progressing and the promotion of the company’s GI product portfolio in the US continues. Our valuation is $518m or $18.3 per ADS ($17.3 per ADS previously).
TALICIA’s NDA was submitted
On 7 May 2019, RedHill announced that it had submitted a new drug application (NDA) to the FDA under the 505(b)(2) regulatory pathway for TALICIA for the treatment of H. pylori infection. The review process should be fast due to the TALICIA’s Qualified Infectious Disease Product designation, which grants priority review with a response expected within six months. This means RedHill is on track to receive a response from the FDA, and perhaps even launch the drug, by end-2019. Our model includes revenues in 2020, but some initial sales could be booked in 2019. If approved, RedHill plans to use its existing commercial platform to market TALICIA to healthcare practitioners and expects a minimal incremental cost of launch (several senior new hires were announced in May). Commercial manufacturing is already in scale-up mode and RedHill is ready to start discussions with payors.
Update on other R&D projects
Following the positive first Phase III with RHB-104 for Crohn’s disease reported in August 2018, RedHill plans to meet with the FDA in mid-2019 to discuss further development. A pivotal Phase III trial with RHB-204 for pulmonary nontuberculous mycobacteria (NTM) infections is expected to start in H219. RedHill is also working on the design of the confirmatory Phase III studies with BEKINDA for gastroenteritis and diarrhoea-predominant irritable bowel syndrome (IBS-D), but no specific timelines have been provided.
Valuation: $518m or $18.3 per ADS
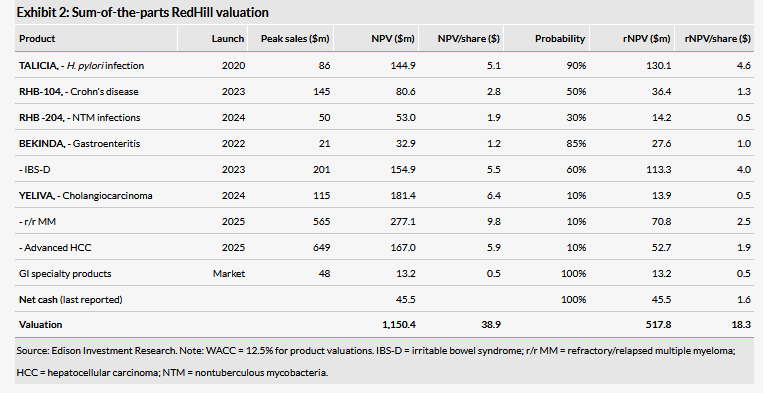
Our revised valuation of RedHill is slightly higher than previously at $518m or $18.3 per ADS vs $491m or $17.3 per ADS. The increase is mainly due to rolling our model forward. At end-Q119, cash and cash equivalents were $45.5m, which should cover RedHill’s operating activities into 2020, according to our model. The potential FDA approval in H219 of TALICIA for H. pylori is the main catalyst in the near term.

Business description
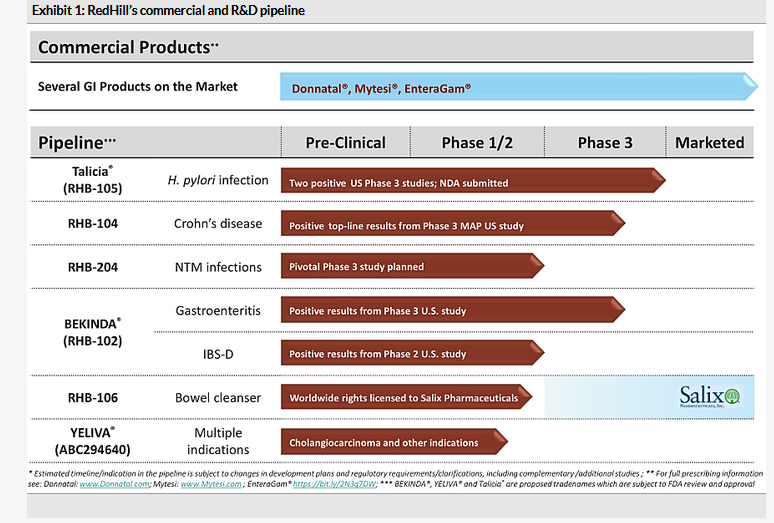
RedHill is a speciality company with an R&D pipeline focusing on gastrointestinal (GI) and inflammatory diseases; earlier-stage assets also target various cancers. The most advanced products are TALICIA for H. pylori infection, RHB-104 for Crohn’s disease, RHB-204 for nontuberculous mycobacteria (NTM) infections and BEKINDA for gastroenteritis and IBS-D. RedHill also promotes four GI products in the US.
Positive results from two Phase III trials with TALICIA
Top-line results from the TALICIA (rifabutin, amoxicillin and omeprazole) confirmatory Phase III study (ERADICATE Hp2) in first-line treatment of H. pylori infection regardless of ulcer status were announced on 3 December 2018. They demonstrate that the primary endpoint was met, which was the H. pylori eradication rate with TALICIA vs active comparator of amoxicillin + omeprazole. The TALICIA treatment group achieved an 84% eradication rate (n=228) vs 58% with the active comparator (n=227), with a high level of significance (p<0.0001). In addition, TALICIA was found to be safe and well tolerated, which is key because the main safety issues seen with rifabutin were not observed in the study. This is likely due to the lower doses used in the study, as concerns about rifabutin toxicity (myelotoxicity) mainly come from treating other infections and using higher doses. The results of this second Phase III study were comparable with the first Phase III ERADICATE Hp study (n=118), which in March 2016 showed that TALICIA eradicated H. pylori in 89.4% of patients (p<0.001). We provided a detailed discussion about the market opportunity for TALICIA in our last update and outlook reports.
R&D pipeline progress
RedHill also provided an update on the remaining projects in its R&D pipeline.
Positive results from the first Phase III with RHB-104 (clarithromycin, rifabutin and clofazimine) for Crohn’s disease were reported in August 2018, which we described in detail in our previous notes. RedHill is assessing additional data and, once finalised, will meet with the FDA, potentially in mid-2019, to discuss further development.
A pivotal Phase III trial with RHB-204 (clarithromycin, clofazimine and rifabutin) for first-line pulmonary nontuberculous mycobacteria (NTM) infections is expected to start in H219. This is still subject to completion of the ‘ongoing supportive non-clinical program’ and additional input from the FDA. The upcoming Phase III study could be sufficient for the approval of RHB-204 as a standalone, first-line treatment for pulmonary NTM infections caused by Mycobacterium avium complex (MAC). From a historical perspective, the NTM infections indication is the latest addition to RedHill’s R&D pipeline and we reviewed the potential of RHB-204 for these difficult-to-diagnose and difficult-to-treat NTM infections in our outlook report.
RedHill is also working on two indications for BEKINDA (bimodal extended release, once-daily, ondansetron) – acute gastroenteritis and diarrhoea-predominant irritable bowel syndrome (IBS-D). The company met with the FDA after the positive results from the first Phase III trial with BEKINDA for gastroenteritis and is now designing a confirmatory Phase III study in this indication. Similarly, RedHill is finalising the design of two pivotal Phase III studies with BEKINDA for IBS-D after a positive Phase II trial. No specific timelines have been provided.
YELIVA (SK2 Inhibitor) is undergoing a Phase IIa study in cholangiocarcinoma, with the study expected to be fully enrolled (n=39) by the end of 2019. In addition, YELIVA is being explored in two other investigator-led clinical trials in refractory/relapsed multiple myeloma and advanced hepatocellular carcinoma. Please refer to our outlook report for more detail on these projects.
Financials
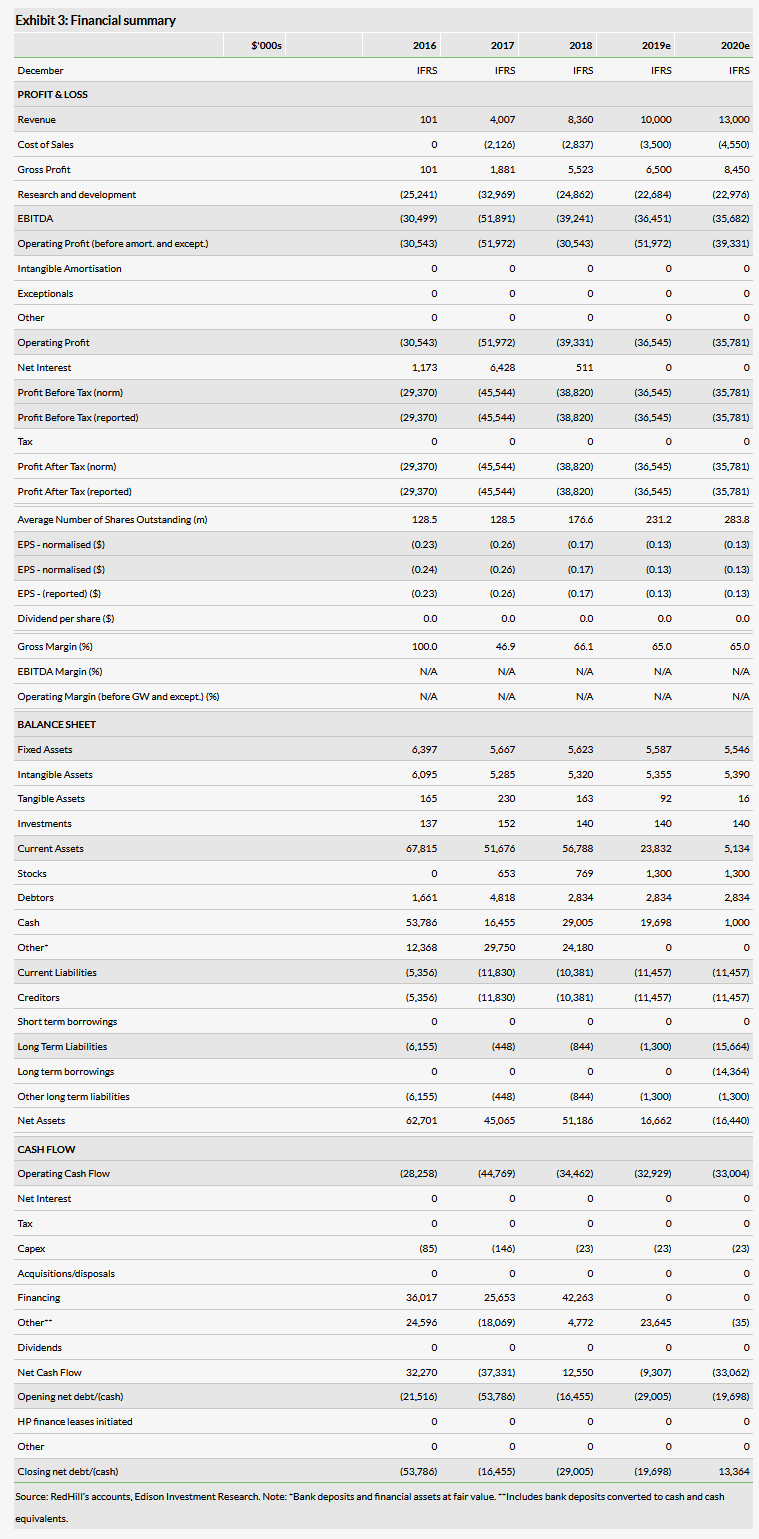
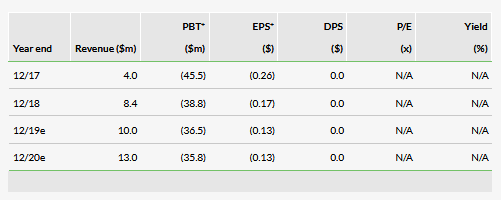
RedHill commercialises and promotes a portfolio of GI products in the US. In Q119 revenues were $1.7m vs $1.4m in Q418 and $2.5m in Q118. 2018 was the first full year that RedHill promoted its GI products, and there is therefore still a limited number of data points for forecasts. The GI products were launched in mid-2017, with the latest addition of Mytesi in June 2018. Total 2018 sales were $8.4m. Q119 sales were lower y-o-y, but higher q-o-q. During a commercial product launch, fluctuations in sales on a quarterly basis are expected. We therefore maintain our growth expectations for 2019 and 2020, but revise our top-line estimates slightly downwards to $10.0m and $13.0m, respectively.
The Q119 gross margin increased to 76% from 66% in FY18. RedHill explained that this was due to a variation in product mix, but provided no specific guidance on a sustainable level going forward. We therefore keep 65% in our model as previously.
The Q119 operating loss was $9.2m, slightly down from $10.0m in Q118, mainly due to lower R&D costs as several large clinical trials ended in 2018, as well as growing sales. We have lowered our 2019 R&D cost estimate as some of the trials are still in the design phase. Our 2019 and 2020 operating loss estimates are now $36.5m and $35.8m, respectively.
The end-Q119 cash and cash equivalents were $45.5m which, as before, should cover RedHill’s operating activities into 2020 according to our model. This includes the potential launch of TALICIA, if the approval process proceeds according to plan.
Valuation
Our RedHill valuation has increased slightly to $518m or $18.3 per ADS from $491m or $17.3 per ADS, mainly due to rolling our model forward. We maintain most of our long-term valuation assumptions, although we have increased the phasing of R&D in our RHB-204 for NTM infections project by one year based on the latest update on when the study could start (described above). Our detailed assumptions for each of the indications are discussed in our last outlook report. Potential FDA approval of TALICIA for H. pylori in H219 is the main catalyst in the near term.