Photocure (LON:0IMT) announced a global licensing deal for Cevira with Asieris, a China-based specialty pharmaceutical company. Cevira is a non-invasive photodynamic therapy for HPV-related (cervical) diseases and has an SPA in place with the FDA. As part of the agreement, Photocure will receive up to $250m (NOK2.1bn) in potential milestones, including $5m (NOK43m) within six months. Royalties will be tiered and range between 10% and 20%. Asieris is expected to launch a Phase III in China initially, with that study expected to complete in 2022. US and EU development will depend on the results of that study.

Business description
Photocure specialises in photodynamic therapy. Its bladder cancer imaging product is sold as Hexvix in Europe and Cysview in the US. It handles the marketing in Nordic countries and the US, while Ipsen is its marketing partner in the EU. Cevira was licensed to Asieris with a Phase III expected to be initiated in the coming months.
Cevira would serve a large market
Cevira is an integrated combination of a drug with an intra-vaginal device for the treatment of patients with HPV-related diseases of the cervix. It has demonstrated statistically significant efficacy in patients with high-grade squamous intraepithelial lesions (HSIL), which has over one million cases diagnosed annually in the US and EU and indicates a higher risk of cancer. In China, 1–2% of women are diagnosed with HSIL every year, indicating a potential market size of 7–14 million.
A deal that retains upside for Photocure
As part of the agreement, Photocure will receive up to $250m (NOK2.1bn) in potential milestones, including a signing fee of $5m (NOK43m) within six months, up to $18m (NOK154m) upon the achievement of certain clinical and regulatory milestones in China and up to $36m (NOK309m) for achieving clinical and regulatory milestones in the US and EU. Royalties will be tiered and range between 10% and 20%.
Phase III development to begin in China
Asieris is expected to launch a Phase III in China initially and include design elements previously agreed upon with the FDA. As a reminder, according to the special protocol assessment (SPA) with the FDA, to gain US approval, two randomised studies with 200 patients each, comparing Cevira to placebo in women with biopsy-verified, high-grade cervical lesions would be needed. The initial Phase III in China is expected to complete in 2022.
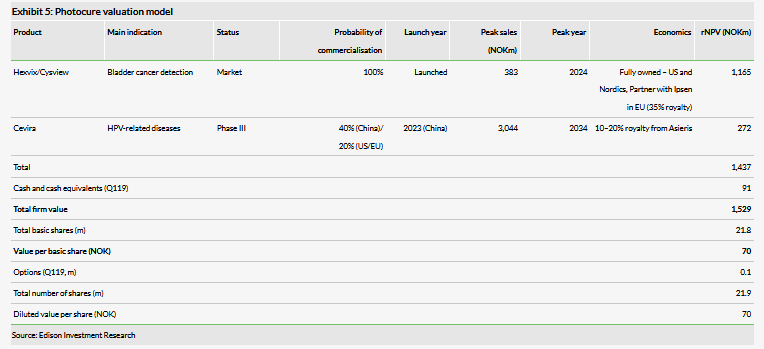
Valuation: NOK1,529m or NOK70 per share
We have increased our valuation to NOK1,529m or NOK70 per basic share, from NOK1,256m or NOK58 per basic share. The increase is due to the inclusion of Cevira in our valuation model following this licensing deal.
Cevira licensed to Asieris
Photocure has announced that it has signed a global licensing agreement with Asieris, a Chinese development-stage specialty pharmaceutical company focused on genitourinary diseases, particularly cancers. As part of the agreement, Photocure will receive up to $250m (NOK2.1bn) in potential milestones including a signing fee of $5m (NOK43m) within six months, up to $18m (NOK154m) upon the achievement of certain clinical and regulatory milestones in China and up to $36m (NOK309m) for achieving clinical and regulatory milestones in the US and EU. A second approved indication in the US, EU and China would result in an additional $14m (NOK120m). Royalties will be tiered and range between 10% and 20%. We view this licensing agreement favourably as the cost of development will be borne by Asieris and hence Photocure can reap rewards with minimal additional investment.
As a reminder, Cevira is a non-invasive photodynamic therapy based on a gel form of hexaminolevulinate hydrochloride (HAL) under development for HPV-related (cervical) diseases and has an SPA in place with the FDA. Cervical cancer is caused by HPV, which can cause normal cells on the cervix to become abnormal. It can take five to 10 years after infection for cells to become abnormal, with abnormal cells graded either as low-grade squamous intraepithelial lesions (LSIL) or high-grade squamous intraepithelial lesions (HSIL). LSIL usually indicates mild dysplasia (normally graded as CIN 1) with a 13% chance of turning into a more severe form of dysplasia (CIN 2/3) over the next two years, according to the American Academy of Family Physicians. Also, only about 2% of patients progress to cervical cancer within 10 years, while 74% regress to normal in five years and 88% regress to normal in 10 years.1 Due to this low risk of progression to cancer and high probability of regression to normal, LSIL is often untreated, with ‘watch and wait’ being the dominant paradigm.
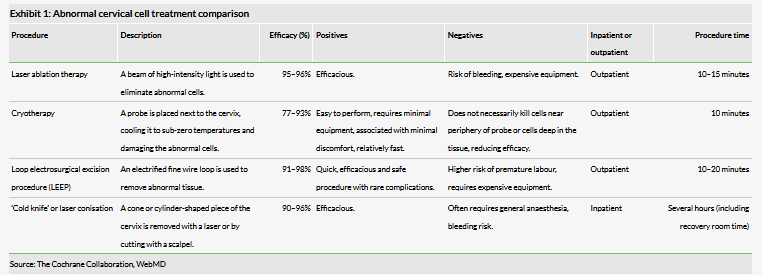
Patients with HSIL have around a 15–20%2 chance of getting cervical cancer and so those patients are usually treated in a number of ways, either by ablative or excisional treatments (see Exhibit 1).
Cevira treatment consists of an HAL gel, along with a disposable battery-powered LED device that is inserted next to the cervix. The HAL gel surrounds the cervix and after five hours, the time it takes for the gel to enter infected cells and be metabolised, then the device is inserted and the device’s LEDs are activated for 4.5 hours. The LEDs then activate the drug and kill the abnormal, precancerous cells (although some normal cells are also killed).
The company ran a 262-patient Phase IIb trial comparing three different concentrations of HAL gel (0.2%, 1% and 5%) to placebo in patients with low to moderate grade cervical intraepithelial neoplasia (CIN 1/2). The primary endpoint was lesion response rate at three months, with a response originally defined as histological regression to CIN 1 or normal, cytology of LSIL or less severe and HPV negative. The 0.2% and 1% doses were no different from placebo, although the 5% dose showed a 73% response in confirmed CIN 1/2 patients vs 60% placebo (p=0.2). However, there was a statistically significant response in the HAL 5% dose patients with confirmed CIN 2. 18 of 19 (95%) patients in the HAL arm compared to 12 of 21 (57%) patients in placebo responded (p<0.001). Importantly, among patients with the oncogenic HPV 16/18 subtypes, which are responsible for 70%3 of cervical cancer cases, HPV clearance was seen in five of six (83%) patients in the HAL arm compared to two of six (33%) in placebo at the six-month point.
Also, at the behest of the FDA, the company conducted a reanalysis of the results, which included a new pathological assessment conducted by a panel of three independent pathologists (originally the samples were only read by one pathologist) and applied new clinical success criteria. As a result of this re-read of the results, 76% of HSIL patients in the Cevira group responded compared to 28% in the treatment arm, a statistically significant difference.
Treatment of abnormal cervical cells related to HPV is a large market. There are 50m pap tests performed each year in the US alone, with approximately 5% returning an abnormal test result. Most of these cases are LSIL, with the number of HSIL cases around 500,000 in the US according to the American College of Pathology, with a similar amount in Europe, making the addressable market around one million in those areas. According to estimates provided by the company, in China, 1–2% of women are diagnosed with HSIL every year, indicating a potential market size of seven to 14 million.
An overview of the Chinese healthcare market
China is the largest pharmaceutical market in the world by volume with a population of 1.4 billion with new policies being adopted across all healthcare market segments to promote improved care, greater innovation and international collaboration. These include initiatives to improve the quality of pharmaceutical products and reduce regulatory bottlenecks.
Reimbursement in China
China’s reimbursement system is almost entirely public, with 97% of individuals covered. Chinese citizens are covered under one of three schemes: Urban Employee Basic Medical Insurance (UEBMI), Urban Resident Basic Medical Insurance (URBMI), or New Rural Cooperative Medical Scheme (NRCMS). To complicate matters further, each of these schemes varies based on local government, with wide variations in benefits. UEBMI is by far the best-funded program and the predominant payer in terms of volume, despite only covering 19% of the population. Reimbursement is 75% for inpatient procedures and drugs, and outpatient costs are typically handled via a medical savings account (MSA), which is mandatory for payees and is financed primarily by payroll taxes.
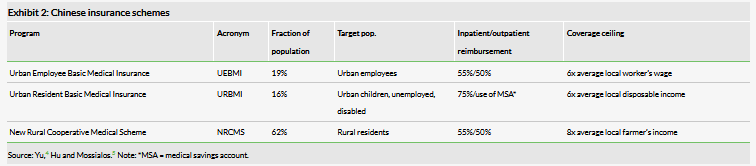
Despite the high number of insured individuals, there are still significant hurdles to receiving care in China. Generally, patients pay for medical procedures upfront then apply for reimbursement, which puts patients with low amounts of disposable income at a significant disadvantage. Additionally, although the NRCMS has had significant success in extending coverage to vulnerable people in China’s countryside, this population continues to have issues with access to quality care.
Historically, deficiencies in the public health insurance infrastructure have been met through out-of-pocket spending. The total out-of-pocket contribution for healthcare costs was 33% in 2011 and the government has stated a goal of reducing this to 30% by 2018. These expenses have been implicated in the exceptionally high rate of household saving in China at 38% in 2014, the highest in the world.6 This savings rate has consistently increased since the early 2000s with the ageing population of China. In a given year, approximately 13% of Chinese households experience a catastrophic medical expense, defined as spending of more than 40% of their disposable income,7 so the need to address significant out-of-pocket medical costs is a common occurrence.
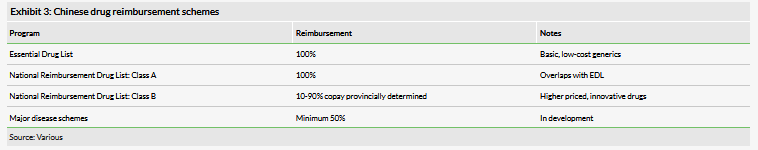
There are several national regulatory schemes in China that determine drug pricing and reimbursement, although they only cover a portion of the drugs that are commercially available in China. The Essential Drug List (EDL) names widely used, low-cost generics that are intended as drugs required for basic care. The National Reimbursement Drug List (NRDL) is a separately administered list of drugs, divided into two parts: Class A for essential generics, which heavily overlaps with the EDL, and Class B, which includes more expensive and non-generic drugs. In theory, drugs on the EDL and NRDL Class A are fully reimbursed, although in practice this is limited by the resources of the individual insurance schemes and local jurisdiction. The NRDL Class B list is reimbursed on a provincial level with copayments of between 10% and 90%. The prices of these drugs also have a high degree of variability compared to their western counterparts, ranging from 30% or less of the US list price for innovative cancer drugs to par for low-cost generics and subsidized programs. A limitation of the NRDL historically has been the frequency at which it was updated: the list received its first revision in eight years in early 2017. The government is also developing the so-called major disease schemes system, which provides reimbursement at a minimum of 50% for patients with certain high-cost conditions such as cancer or autoimmune disorders. These programs are still in the pilot stages.
Recent regulatory changes
One of the biggest focuses of regulatory reform in the Chinese healthcare system has been improving the availability of innovative medicines. A major limiting factor in the approval of new drugs in China has been the regulatory backlog. Historically, a new drug application was filed each time a manufacturer launched a competing generic and there was no apparatus to effectively identify which applications should receive priority review. As of 2014 there were approximately 19,000 open drug applications, and fewer than 100 employees involved in their review at the China Food and Drug Administration (CFDA). In efforts to reduce the backlog, the agency has increased the number of reviewers to 600 and reduced the number of outstanding applications to approximately 4,000 by the end of 2017.8
The agency has also instituted a series of pathways to market to expedite the approval of innovative drugs. In particular, these new policies open up the process to drugs that have been approved by foreign regulatory agencies. In June 2017, the CFDA joined the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use, the organization tasked with standardizing drug approval standards across regulatory agencies. To facilitate the approval of foreign medicines and align its process with foreign agencies, the CFDA created a series of new drug classifications, which take into consideration the approval status of a drug overseas and reduce the clinical requirements for drugs that have been approved elsewhere. However, in October 2018, the State Council announced a draft proposal that would further reduce the clinical burden for imported drugs by allowing the CFDA to accept overseas clinical trial data as part of Chinese application packages. The proposal included the requirement that application includes clinical data on ‘the existence of ethnic differences,’ presumably to ensure similar activity in Chinese populations. This requirement is in accordance with the historical motivation for requiring additional Chinese clinical trials. The degree of implementation of this policy, or the precise requirements for foreign data are unclear, as the draft proposal did not include specifics or a timeline for implementation. The extent to which these policies will differ between innovative drugs and generics is also unclear. Additional reforms to encourage the import of medicines were announced at the meeting of the State Council in April, including the removal of import tariffs and reduced VAT on ‘common drugs’ including all anticancer drugs.
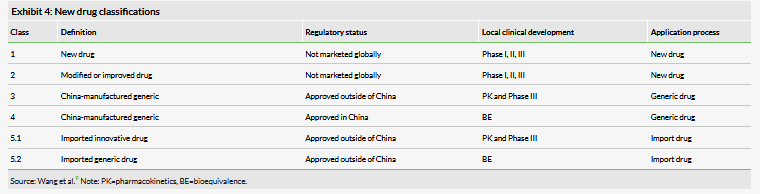
The agency has set up a series of criteria for priority review to shorten the time to approval for new drugs. According to the agency’s most recent report on the program, it takes approximately 39 days to process a priority CTA, 59 days for an NDA and 81 days for ANDA. Priority review is awarded to:
- innovative drugs not approved elsewhere,
- innovative drugs that will be manufactured in China,
- innovative drugs for HIV, hepatitis, rare diseases, malignant tumours and paediatric diseases, among others, and
- newly launched generics.
China is also reforming its approach to intellectual property with regard to pharmaceuticals. It is moving to a patent-linkage system similar to that present in the US, where a generic applicant must reference the originator patent and inform the holder, thus initiating an appeals process. The CFDA also proposed a series of data exclusivity periods for different classes of drug: six years for an innovative small molecule and 12 for a biologic.
Finally, regarding regulatory reforms, the Chinese government announced in March 2018 that the CFDA, as well as other healthcare agencies, would be reorganised into a larger market regulatory body. We expect this reorganisation to increase governmental pressure on the agency, but that most of the CFDA’s previous mandate will remain intact.
Valuation
We have increased our valuation to NOK1,529m or NOK70 per basic share, from NOK1,256m or NOK58 per basic share. The increase is due to our inclusion of Cevira in our valuation model following this licensing deal, which we had recently removed as the program had been on hold for over five years.
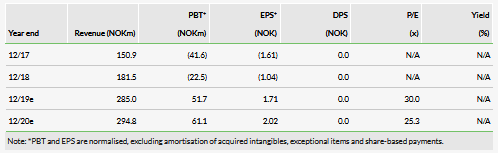
For the US/EU, we are projecting a launch in 2025 and 6% peak market share at a price of approximately NOK9,400 per patient at launch. We currently apply a 20% chance of success for the US and EU markets, a significant discount to our typical 60–70% probability of success for a Phase III asset due to the significant unknowns related to timing and the ability of Asieris to finance and execute a clinical development plan. For China, we are projecting a 2023 launch with 3% peak market share and a NOK4,300 price per patient at launch. We apply a 40% probability of success in that region, which continues to be a discount to our typical probability of success due to the uncertainties related to Asieris. It is, however, larger than our probability of success for the US and EU as development is starting in China and hence there are fewer unknowns. We view our Cevira estimates as conservative, with upside potential once the program progresses through development.
Financials
The company ended Q119 with NOK91m in cash. We have added NOK43m ($5m) in licensing revenues for 2019 due to the expected payment from Asieris, otherwise our financial estimates remain the same. We do not expect Photocure to require further financing as we continue to forecast profitability in 2019.