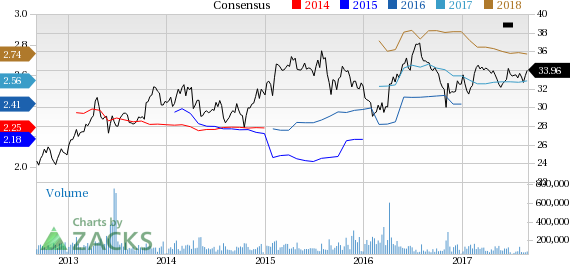
Pfizer Inc. (NYSE:PFE) announced that the FDA has granted approval to Mylotarg for adult patients with newly diagnosed CD33-positive acute myeloid leukemia (AML). The drug also received approval to treat relapsed or refractory CD33-positive AML in adults as well as children (2 years and older).
The approval was expected as the FDA’s Oncologic Drug Advisory Committee had voted in favor of Mylotarg’s approval in July 2017.
However, it should be noted that the U.S. label of the drug will include a boxed warning for hepatotoxicity including severe or fatal hepatic veno-occlusive disease.
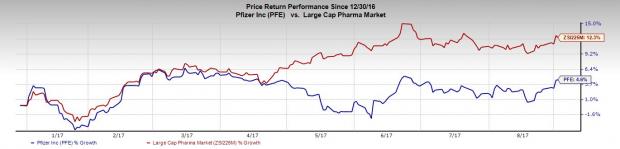
Pfizer’s shares have gained 4.6% so far this year, underperforming the industry’s increase of 12.3%.
The approval was granted based on data from multiple investigator-led clinical trials, including ALFA-0701, AML-19 and MyloFrance-1.
The ALFA-0701 phase III study evaluated Mylotarg in combination with chemotherapy in newly diagnosed AML patients. The drug showed significant improvement in event-free survival (EFS), achieving 17.3 months compared to 9.5 months for chemotherapy.
The phase III AML-19 study compared Mylotarg as monotherapy with best supportive care in elderly AML patients who are intolerant to other therapies. Mylotarg achieved a better median overall survival period of 4.9 months versus 3.6 months for best supportive care.
MyloFrance-1, a phase II study, evaluated single agent Mylotarg in relapsing AML patients. The study showed that almost 26% of patients achieved complete remission and the median relapse-free survival achieved was 11.6 months.
It is evident from the above studies that Mylotarg’s achievements support its risk benefit profile in AML patients.
We remind investors that Mylotarg was granted accelerated approval by the FDA in 2000 as a single agent for treating CD33-positive AML in patients who had experienced their first relapse and were 60 years or older. However, 10 years later, Pfizer voluntarily removed Mylotarg from the market as data from a post approval phase III study, SWOG S0106, showed no clinical benefit and rate of fatalities due to treatment-related toxicity was significantly higher.
Per the press release, CD33 antigen occurs in nearly 90% of AML patients. In fact, AML occurs in nearly 80% of acute leukemia patients. Moreover, Mylotarg is the first approved therapy for pediatric AML, which will give Mylotarg first-mover advantage. An estimated 21,380 patients in the United States are expected to be diagnosed with AML in 2017 including 500 children, as per the data published in the press release.
Chemotherapy is the current preferred therapy for treating AML. With Mylotarg’s approval a new therapy with improved benefits will be available to patients. However, several other companies are also developing therapies for treating AML. These include Seattle Genetics, Inc.’s (NASDAQ:SGEN) SGN-CD33A and Intrexon Corporation’s (NYSE:XON) CD33-specific CAR+ T therapy. In August 2017, the FDA approved Celgene Corporation’s (NASDAQ:CELG) Idhifa as an oral treatment for relapsed or refractory AML patients.
Pfizer currently has Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Zacks' 10-Minute Stock-Picking Secret
Since 1988, the Zacks system has more than doubled the S&P 500 with an average gain of +25% per year. With compounding, rebalancing, and exclusive of fees, it can turn thousands into millions of dollars.
But here's something even more remarkable: You can master this proven system without going to a single class or seminar. And then you can apply it to your portfolio in as little as 10 minutes a month.
Pfizer, Inc. (PFE): Free Stock Analysis Report
Celgene Corporation (CELG): Free Stock Analysis Report
Seattle Genetics, Inc. (SGEN): Free Stock Analysis Report
Intrexon Corporation (XON): Free Stock Analysis Report
Original post
Zacks Investment Research