At ASH (American Society of Hematology) this weekend, Novartis (NYSE:NVS) presented data from its global registration trial (ELIANA) of CTL019 in relapsed/refractory (r/r) paediatric and young adults with B-cell acute lymphoblastic leukaemia (B-ALL). In one of the largest CAR-T trials to date, 41 out of 50 (82%) treated patients achieved a complete remission or complete remission with incomplete blood count recovery.
These positive results have prompted Novartis to reaffirm its timelines for filing in B-ALL, with a submission to the FDA expected in early 2017 and to the EMA later in 2017. This data, along with the restated timelines, reinforce our forecasts for Oxford Biomedica (LON:OXB), which manufactures and supplies the vectors that are utilised in CTL019. We value OXB at £173m (6.2p/share).
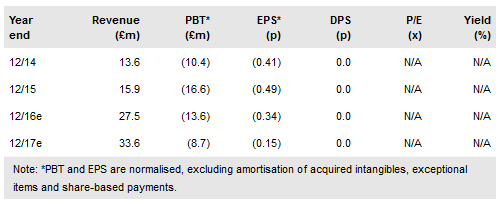
The positive data for CTL019 mean we retain our expectations for a launch in 2017, from which OXB will generate revenues (milestones, manufacturing and royalties) from the production of its lentiviral vectors, which are a key component of CTL019. Efficacy data from the ELIANA trial was positive, no minimal disease was detected in any of the 41/50 patients (82%) who achieved complete remission. While a key concern for CAR-Ts in general has been duration of response, Novartis reported an impressive estimated relapse-free rate of 60% six months after the administration of treatment. Additionally, following the setbacks to Juno’s CD19 CAR-T following the death of a further two patients following neurotoxicity, it is positive to see that Novartis reported no grade 4 or above neurotoxic events. In line with most CAR-Ts to date, 48% of patient’s experienced grade 3 or 4 cytokine release syndrome. Novartis reported no patient deaths relating to treatment. We maintain our value of OXB at £173m (6.2p/share) as timelines and data are in line with our previously published forecasts.
To read the entire report Please click on the pdf File Below