Novartis AG (NYSE:NVS) announced new data on chronic spontaneous urticaria (CSU) drug Xolair at the 26th European Academy of Dermatology and Venereology Congress in Geneva, Switzerland from a phase IIIb study, OPTIMA.
OPTIMA is a phase IIIb international, multicenter, randomized, open-label, non-comparator study. 314 patients with CSU symptoms despite treatment with H1-antagonists were initially randomized 4:3 to Xolair 150 or 300 mg for 24 weeks in the first dosing period. Thereafter, patients who responded well to the treatment were given a break and then, if symptoms returned were retreated.
Per the data, 90% of CSU patients who responded well to initial treatment with Xolair regained symptom control within 12 weeks following a treatment interruption based on Weekly Urticaria Activity Score (UAS7) criteria (UAS7=
We note that Xolair is approved for the treatment of CSU in the EU and the United States (chronic idiopathic urticaria). Xolair is also approved for the treatment of moderate-to-severe or severe persistent allergic asthma.
Novartis has a collaboration agreement with Roche Holdings AG (OTC:RHHBY) to develop and co-promote Xolair in the United States.
We note that Novartis is a global leader in Immunology & Dermatology with drugs like Cosentyx, Xolair, Zortress and Myfortic and Ilaris.
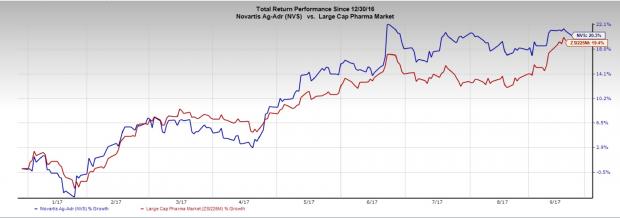
Novartis’ stock has rallied 20.3% in the year so far compared with the industry’s 19.4% gain.
Novartis is currently going through a transitional stage. The company’s blockbuster drug, Diovan, is facing stiff generic competition in the United States, EU and Japan. Gleevec lost exclusivity in the United States in February 2016. The company also lost patent protection for the drug in EU in December 2016 leading to generic competition.
The loss of patent protection for these top-selling drugs continue to hurt sales. Hence, Novartis expects the next growth phase to begin in 2018 driven by Cosentyx (in psoriasis, psoriatic arthritis and ankylosing spondylitis indications), Entresto, Kisqali and a deep pipeline with candidates like BAF312, AMG 334, RTH258.
Moreover, with the recent approval of Kymriah, Novartis is the first company to bring CAR-T cell therapy for cancer treatment. Going forward, we expect that the approval of new drugs and label expansion of existing ones to bode well for Novartis.
On the other hand, generic division, Sandoz is a leader in biosimilar market with five marketed biosimilars (Omnitrope, a human growth hormone; Binocrit, an erythropoiesis-stimulating agent used to treat anemia; and filgrastim for neutropenia under the brand names Zarzio outside the United States and Zarxio in the United States.).
The company plans to launch five major oncology and immunology biosimilars between 2017 and 2020. This includes a biosimilar version of Rituxan (rituximab), which was approved by the European Commission in June 2017 (marketed as Rixathon). In August 2016, Sandoz’s Erelzi, a biosimilar version of Amgen, Inc.’s (NASDAQ:AMGN) blockbuster drug Enbrel gained approval in the United States for five indications. Erelzi was also approved by the European Commission in 2017.
Zacks Rank & Stock to Consider
Novartis currently carries a Zacks Rank #3 (Hold).
Aduro Biotech, Inc. (NASDAQ:ADRO) is a better-ranked biomedical company with a Zacks Rank #2 (Buy). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Aduro’s loss estimates narrowed 9.6% to $1.32 for 2017 and 20% to $1.24 for 2018 over the last 60 days. The company delivered an average earnings beat of 2.53% for the four trailing quarters.
New Report: An Investor’s Guide to Cybersecurity
Cyberattacks have become more frequent and destructive than ever. In fact, they’re expected to cause $6 trillion per year in damage by 2020. The cybersecurity industry is expanding quickly in response to these threats. In fact, a projected $170 billion per year will be spent to protect consumer and corporate assets. Zacks has just released Cybersecurity: An Investor’s Guide to Locking Down Profits, which reveals 4 promising investment candidates.Download the new report now>>
Roche Holding (SIX:ROG) AG (RHHBY): Free Stock Analysis Report
Novartis AG (NVS): Free Stock Analysis Report
Amgen Inc. (AMGN): Free Stock Analysis Report
Aduro Biotech, Inc. (ADRO): Free Stock Analysis Report
Original post
Zacks Investment Research
