Newron Pharmaceuticals' (SIX:NWRN) interim results highlight that despite additional worldwide launches of Xadago, sales growth in the US and Europe remain below our expectations. For Evenamide, the company is addressing FDA concerns on the pre-clinical data, and plans are underway for short-term explanatory studies prior to starting the pivotal Phase III trials. Top-line data from the pivotal trial STARS, investigating sarizotan in Rett syndrome, is expected in Q419. The reported net loss increased 85% to €14.0m in H119, driven by higher than expected net R&D expenses. Reassessing our sales expectations for Xadago and operating forecasts, we now value Newron at CHF466m from CHF653m.
Xadago ramp up required
Newron reported €2.2m in Xadago/safinamide related royalties in H119 (vs €2.0m in H118). We have previously highlighted that a ramp up in sales is necessary if this asset is to reach our prior forecasted global peak sales of €672m (in PD alone). Based on our analysis of prescription trends we feel it prudent to revise our peak sales estimates down to €394m. Partner Zambon will initiate a label extension study for Xadago in levodopa (L-DOPA) induced dyskinesia (LID) in H219; we continue to include a risk-adjusted contribution for Xadago in LID, assuming peak sales of €364m ($400m). This study could differentiate Xadago in a genericised PD market, particularly in the US.
STARS data key inflection, Evenamide trials delayed
The pivotal Phase II/III Sarizotan Treatment of Apnoeas in Rett Syndrome (STARS) trial is expected to report top-line data in Q419 potentially supporting an NDA filing in 2020; accelerated review is also possible (six months). The Phase III programs for Evenamide in schizophrenia have been delayed until the FDA’s concerns on preclinical data are addressed; we anticipate more guidance before year-end.
Financials: Cash reach extended through EIB facility
The net loss widened in H119 due to increased net R&D expenses (€10.3m, +105%) relating to STARS and Evenamide’s pivotal studies. The first €10m tranche from its loan facility with the EIB provides Newron funding into FY21. Drawing down additional tranches (up to €30m) could extend this further.
Valuation: CHF466m or CHF26.1/share
Our revised valuation of Newron is CHF26.1/share from CHF36.6/share, reflecting a significant reduction in our forecasted Xadago PD sales; our LID forecasts remain unchanged. Additionally, we rolled forward our model and updated FX rates and net cash and short-term investments at 1 July 2019 of €29.9m.
Business description
Newron Pharmaceuticals is an Italian CNS-focused biotechnology company. Xadago (safinamide) for Parkinson’s disease has been launched in Europe and the US. Xadago is partnered with Zambon (EU), Meiji Seika (Japan), US WorldMeds (US), Seqirus (Australia/New Zealand) and Medison Pharma (Israel).
Xadago sales forecasts revised, sarizotan launch on the horizon
Xadago launched in Europe during H115 and in the US during H217, as an add-on (adjunctive) treatment for patients with Parkinson’s disease (PD) on levodopa (L-DOPA) experiencing treatment related motor symptoms (ON/OFF effect). Reported royalties in H119 of €2.2m (H118: €2.0m) are disappointing, implying net sales only grew 8% to c €27m (H118: c €25m); we assume a royalty rate varying between 8–15% depending on country and partner. In Europe, where Xadago is being commercialised by partner Zambon, Newron reported single-digit growth. Xadago (safinamide) has multiple mechanisms of action, which could set it apart from other adjunct monoamine oxidase inhibitors, such as rasagiline and selegiline; however, these are priced substantially cheaper (c 3–14% compared to Xadago) and without directly head-to-head efficacy studies or a label extension into LID (which could differentiate it), achieving significant sales growth and market penetration in Europe could be challenging, even with the reimbursement cap in Italy lifted Q219. Although Newron and Zambon could consider discounting to increase volumes, we expect there is a reluctance to do this as it would hinder future pricing strategies if a label extension is achieved.
The US market is critical, with sales through sub-licensee US World Meds contributing c 70 % of our forecasted peak sales. As highlighted in Exhibit 1, new prescriptions (NRx) in the US appear to be relatively flat since Q218 although gross sales grew c 41% during H119 (c $7.2m), should NRx continue to grow at this rate, growth in total prescriptions (TRx) will quickly begin to plateau. Based on our previous forecasts, we had anticipated US sales in 2019 would have grown more significantly. In light of this US prescribing data and the underperformance in Europe, we have reduced our global peak sales expectations for Xadago in PD to €394m (from €679m) and will continue to monitor this sales evolution closely. We believe that broader clinical adoption, particularly by US prescribers, will be required to before meaningful sales momentum can be garnered.
Exhibit 1: Estimated Xadago US quarterly gross sales
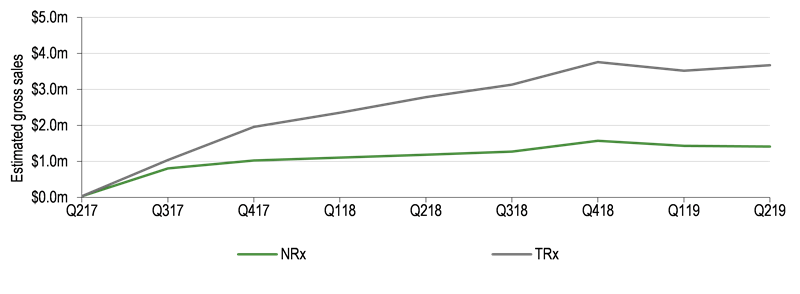
Post-period, partners Meiji Seika and Eisai received marketing approval for safinamide from the Japanese regulators. The drug will be marketed as Equifina, indicated as adjuvant treatment for PD patients on levodopa experiencing the wearing-off phenomenon. We anticipate a commercial launch during 2020, but expect royalties received to provide a minor contribution to reported revenues in both the near and long term.
Label extension study in LID could be a differentiating catalyst
Newron and partner Zambon are due to initiate (H219) a pivotal efficacy study to support Xadago use in levodopa (L-DOPA) induced dyskinesia (LID). We believe this single Phase III trial could support a label extension, which in a highly genericised PD market could help differentiate Xadago from all other classes of PD drug treatments. It is estimated that dyskinesia affects around 40% of PD patients treated with L-DOPA for four to six years, with limited treatment options aside from LDOPA dosing adjustment. With around one million PD patients in each of the US and Europe, this represents a large opportunity. Newron expects having dyskinesia on the label will enable Xadago to be used earlier in the treatment paradigm and could launch in 2021. We include a separate risk-adjusted contribution for Xadago in LID, assuming peak sales of €364m ($400m). Until there is more clarity on the potential magnitude of benefit in this indication, our LID forecasts remain unchanged, but we will review our assumptions once more details on the trial design have been announced. Newron will co-fund the study up to a capped investment (c €10m), and in return will receive an increased share of the economics under the licence agreement with Zambon.
Sarizotan launch 2021: A breakthrough opportunity in RS
With patient enrolment complete in Newron’s pivotal STARS trial and over 130 patients recruited, Newron has been a pioneer in developing a pharmacotherapy for Rett syndrome (RS), being the first to conduct a Phase III study; top-line data are expected in Q419. Newron is planning to apply for a global filing and approval strategy once STARS data are available, if positive. If approved, sarizotan will be the first drug for RS to reach the market and Newron will commercialise this asset alone in the US with a small but targeted salesforce; a European and broader commercialisation strategy is yet to be defined.
Although Newron is the first to embark on a Phase III study for a pharmacotherapy in RS, Neuren Pharmaceuticals has partnered with ACADIA and plans to initiate a Phase III study (LAVENDER) for its lead asset trofinetide as a treatment for RS in Q419. We note that trofinetide elicits its effect through a distinctly different mechanism of action to sarizotan. Given the underlying pathology of RS is not well defined, we believe it is far too early to ascertain which could be more beneficial to RS patients. Importantly RS is a genetic condition; mutations to the methyl CpG binding protein 2 (MECP2) gene are indicative of the disease in c 95% of cases. Thus gene therapies correcting for this aberration have the potential to provide a curative treatment for RS patients. AVXS-201, an AAV-based gene therapy being developed by AveXis (now part of Novartis), was due to start clinical development during 2019 potentially enabling a regulatory filing in c 2022. However, with the recent fall-out regarding the discrepancies within the data package AveXis/Novartis presented to the FDA for Zolgensma (AVXS-101), the US regulators are requiring additional pre-clinical work to be conducted for AVXS-201, delaying an IND until mid-2020. Given the early stages of development of gene therapies for RS, we have not modelled how paradigm shifts might unfold, but highlight that a curative treatment could render non-disease modifying drugs, such as sarizotan, redundant in the long term.
Both our penetration and pricing assumptions are conservative and we highlight that these will ultimately depend on sarizotan’s magnitude of benefit (demonstrated in the ongoing STARS trial) and outcomes from a separate ongoing BOI study (health economics and outcome research study), which Newron is sponsoring in parallel to STARS. The objective of the BOI study is to better understand the relative burden of each symptom in this multi-symptom disease and quantify how breathing abnormalities affect the quality of life in patients with RS. This study enables Newron to foster partnerships and collaborations with Rett advocacy groups, thought-leading physicians and governing payers. By identifying the unmet need for improving RS disease management and aligning economic and clinical outcomes, the company believes this study will aid in pricing reimbursement discussions, access and market take-up of the drug once the approval process has been initiated.
Evenamide’s blockbuster potential moves further away
Newron's novel mechanism of action drug, Evenamide, was due to enter pivotal clinical development for schizophrenia but experienced a significant set-back during H119. Specifically, the decision by the US regulators to delay the start of pivotal studies, stemming from the emergence of safety concerns in (preclinical) chronic toxicity study in rats and CNS events observed in dogs at high doses, which could be related to a class effect for the drug. Discussions are ongoing with the FDA to discuss what is required in more detail, but management has indicated that additional preclinical and clinical safety studies (presumably Phase I in healthy volunteers) are likely to be required before a Phase III study is cleared by the regulators.
This set back was unexpected given that positive clinical safety data were reported from the Phase I/IIa proof-of-concept study of Evenamide (March 2017). The likely requirement to conduct another Phase I clinical study (50–60 healthy volunteers), even in parallel with preclinical work, will significantly affect development timelines; we note that the original Phase I study for Evenamide (conducted in 54 healthy volunteers) took 18 months to complete. With this in mind, we estimate that registrational studies will not start until late-2020/early-2021, delaying a launch until at least early-2024.
Valuation
Our revised valuation of Newron is CHF466m (CHF26.1/share), versus CHF653m (CHF36.6/share) previously. Our sum-of-the-parts valuation consists of contributions from Xadago sales in Parkinson’s disease (PD), in addition to risk-adjusted contributions from Xadago for PD-related levodopa-induced dyskinesia (LID), sarizotan in Rett syndrome (RS) and Evenamide in schizophrenia. Downgrading our sales expectations for Xadago in PD has had a significant impact on our valuation, given this had previously contributed 48% (CHF17.6/share), now reduced to 35% (CHF9.2/share). We also have rolled forward our model, and updated FX rates and reported net cash of €29.9m at 1 July 2019. The breakdown of our rNPV valuation, which uses a 12.5% discount rate for clinical-stage assets and a 10% discount rate for commercial assets (Xadago in PD), is shown in Exhibit 2.
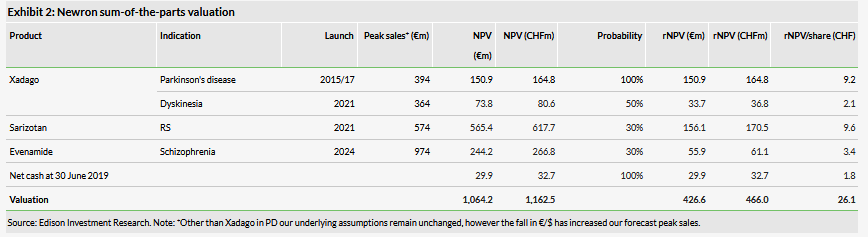
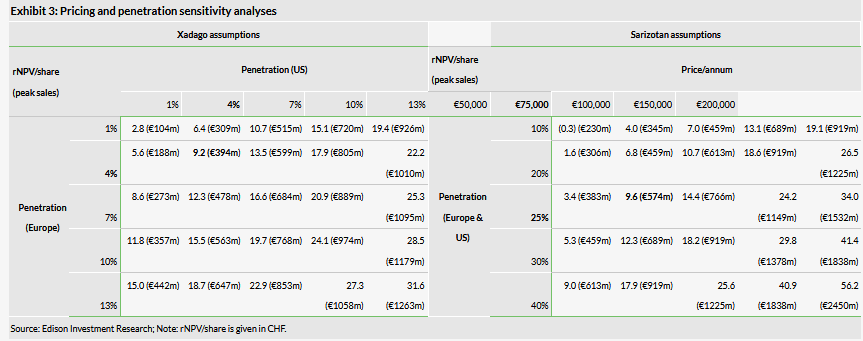
With Xadago in PD and sarizotan in RS comprising 72% of our valuation, there is a significant degree of sensitivity surrounding our base-case assumptions for both drugs; failure for either to reach (or exceed) our peak sales forecasts would materially affect our valuation of Newron. Sarizotan’s contribution to the valuation reflects the potential higher pricing assumption and high operating margin of this asset, which Newron can commercialise alone with a small but focused salesforce (25–30 medical liaison managers). Exhibit 3 shows the penetration and pricing sensitivity; for Xadago in PD (excluding LID), we have reduced our assumed peak penetration from 7% to 4% to attain an rNPV of CHF9.2/share.
Financials
Reported revenues grew 10% during H119 to €2.2m (H118: €2.0m), but the significant uplift in operating expenses (€16.7m, +70%), primarily due to a doubling of net R&D expenditure during the period (€10.3, +104%), led to an increase in reported net loss (€14.0m, +85%). We have reviewed our FY19 estimates and now forecast Newron to report an operating loss of €23.5m in FY19 (vs €14.3m previously).
We have decreased our revenue expectations for FY19 and FY20 revenue to €5.5m and €11.9m, respectively, from €8.6m and €21.7m previously; our near-term revenue forecasts are based primarily on royalty income related to Xadago sales in Europe and the US; these have been downgraded in light of the sustained underperformance of sales in these regions. We also include a €4.0m development milestone from Meiji Seika for regulatory approval in Japan in our 2020 revenue forecast.
Our revised estimate for net R&D expenditure has increased to €19.3m from €13.2m in FY19 and €17.4m from €15.6m in FY20. Reported net costs in H119 were higher than we had previously expected, due to an increase in gross expense relating to STARS and Evenamide’s now delayed pivotal studies, amalgamated by the implementation of the 2019 stability law in Italy (1 January 2019), which has caused a reduction in proportional R&D tax credit received; as a proportion of gross expenditure, the tax credit reduced by 12.4pp to 21.9% during H119. The remaining €15.4m in tax credit on the balance sheet will aid Newron’s operating result in the coming years.
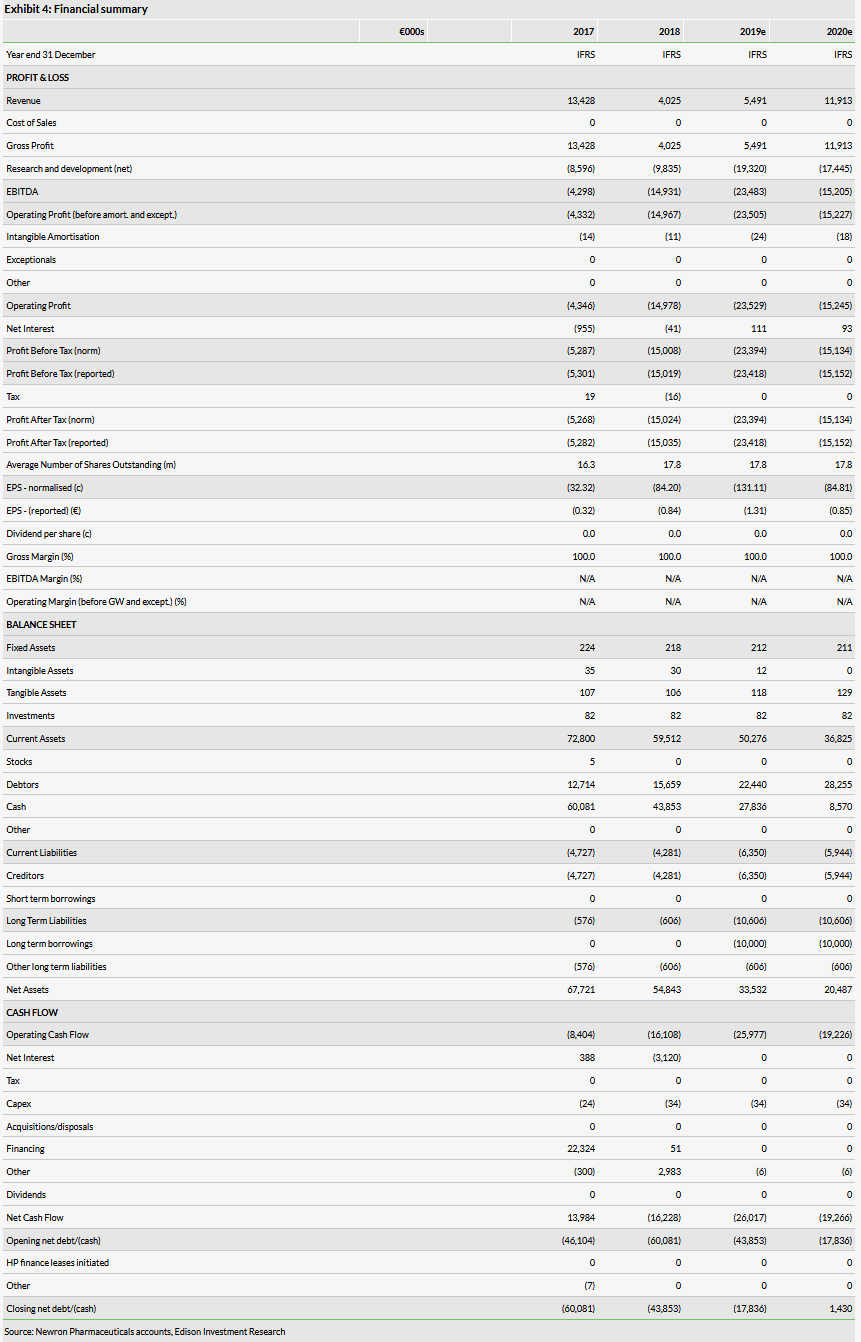
Newron reported cash and equivalents of €39.4m at 30 June 2019, including the first €10m tranche from the EIB loan facility which was booked as a short-term receivable before it was deposited into the company accounts (1 July 2019), when we approximate net cash was €29.9m. We continue to expect that its current cash resources should be sufficient to fund Newron into FY20 through key inflection points given the company has access to a further €30m from the EIB to fund future operations.