Shares of Mylan N.V (NASDAQ:MYL) have declined 9.7% in after-market trading, after the company reported lower-than-expected results for the fourth quarter and issued a tepid 2019 guidance.
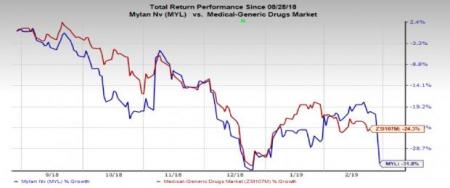
Mylan’s stock has lost 31.8% in the past six months compared with the industry’s decline of 24.3%.
Adjusted earnings of $1.30 per share missed the Zacks Consensus Estimate of $1.33 and declined from $1.43 in the year-ago quarter.
However, fourth-quarter revenues of $3.08 billion missed the Zacks Consensus Estimate of $3.12 billion and declined 4.9% from the prior-year quarter.
Quarter in Detail
The company posts results in three segments on a geographic basis, namely North America, Europe and Rest of World.
North America segment’s net sales came in at $1.10 billion, down 16%. This decline was primarily attributable to lower volumes on existing products due to actions associated with the restructuring and remediation activities at the Morgantown plant, the timing of purchases of products by customers and the impact of the implementation of new accounting standards.
The FDA completed an inspection at Mylan's plant in Morgantown, WV, in 2018 and made observations through a Form 483. Thereafter, Mylan submitted a comprehensive response to the FDA. During the second quarter of 2018, Mylan started a restructuring and remediation program to reduce complexity at the Morgantown manufacturing facility, which led to the discontinuation and transfer of a number of products to other manufacturing sites, a reduction in workforce and extensive remediation activities.
These actions have led to a temporary disruption in supply of certain products. In the fourth quarter, the company received a warning letter related to the previously disclosed observations.
Net sales in the Europe segment were $1.09 billion, up $15.8 million, which was primarily driven by new product sales and higher volumes on existing products.
Rest of World segment’s net sales of $851.4 million were up 4%, driven by new products.
Adjusted gross margin of 54.6% was slightly down from 55.5% in the year-ago quarter.
2018 Results
Earnings per share of $4.58 were up from $4.56 in 2017 but missed the Zacks Consensus Estimate of $4.63. Revenues were $11.43 billion, down 4% from 2017 and missed the Zacks Consensus Estimate of $11.46 billion.
2019 Guidance
Revenues are projected between $11.5 billion and $12.5 billion, with a midpoint of $12 billion that is above the Zacks Consensus Estimate of $11.89 billion.
The company anticipates adjusted EPS around $3.80-$4.80, lower than the Zacks Consensus Estimate of $4.99.
Our Take
Mylan’s fourth-quarter results were disappointing as the company missed on both earnings and sales. While the generic market in large has been experiencing a slowdown, Mylan itself proactively discontinued a number of products along with transferring some to other sites.
These have led to a temporary disruption in supply of certain products and reduced volumes in North America generic sales, including the EpiPen auto injector. We note that rival Teva Pharmaceutical (NYSE:TEVA) has already won the FDA approval for the first generic version of Mylan’s EpiPen and EpiPen Jr, which, in turn, will impact sales further.
Moreover, slower-than-expected uptake of generic Copaxone, even after reducing the price by more than 60%, adversely impacted sales in North America.
The guidance for 2019 was disappointing as well.
Nevertheless, Mylan received a significant boost when the FDA approved its generic version of GlaxoSmithKline’s (NYSE:GSK) Advair Diskus — Wixela Inhub — following a few setbacks. Notably, this is the first generic of Advair Diskus approved, which, in turn, will give Mylan an edge over its competitors. Mylan has launched the generic and continues to gain traction with its biosimilar portfolio.
Mylan and partner Biocon obtained the FDA approval for Fulphila, a biosimilar of Neulasta. The company earlier received the FDA approval for a biosimilar version of Roche Holdings’ (OTC:RHHBY) Herceptin.
Zacks Rank
Mylan currently carries a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Is Your Investment Advisor Fumbling Your Financial Future?
See how you can more effectively safeguard your retirement with a new Special Report, “4 Warning Signs Your Investment Advisor Might Be Sabotaging Your Financial Future.”
Roche Holding (SIX:ROG) AG (RHHBY): Free Stock Analysis Report
GlaxoSmithKline plc (GSK): Free Stock Analysis Report
Teva Pharmaceutical Industries Ltd. (TEVA): Free Stock Analysis Report
Mylan N.V. (MYL): Free Stock Analysis Report
Original post
Zacks Investment Research
