Inovio Pharmaceuticals, Inc. (NASDAQ:INO) reported loss of 31 cents for the first quarter of 2017, narrower than the Zacks Consensus Estimate of a loss of 34 cents. However, the reported figure was significantly wider than the year-ago loss of 11 cents.
Shares of Inovio increased more than 2% in after-hours trading. However, shares have underperformed the Zacks classified Medical-Biomed/Genetics industry so far this year. The stock lost 8.0% during the period, while the industry registered an increase of 2.8%.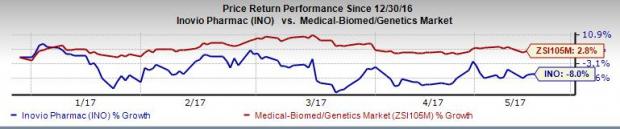
Quarter in Details
Inovio reported revenues of $10.4 million in the first quarter, which comfortably surpassed the Zacks Consensus Estimate of $4 million. Moreover, revenues grew 28.4% from the year-ago period. The top-line improvement is mainly attributable to revenues recognized from the termination payment received from Roche Holding (SIX:ROG) AG (OTC:RHHBY) during the first quarter. In 2016, Roche discontinued its collaboration with Inovio for the development of INO-1800, a hepatitis B DNA immunotherapy.
During the quarter, revenues under collaborative research and development arrangements surged 138% to $4.3 million from $1.8 million a year ago.
Research and development expenses increased 34.6% to $24.5 million due to higher investment in all product development programs.
General and administrative expenses increased 44.4% to $7.8 million, primarily due to an increase in non-cash stock based compensation costs.
Pipeline Update
VGX-3100, an HPV immunotherapy, is the most advanced candidate in the company’s pipeline that is being developed for the treatment of HPV-16/18-related high-grade cervical dysplasia.
In Jan 2017, the company announced a collaboration and license agreement with China’s ApolloBio Corporation to exclusively develop and commercialize VGX-3100 in Greater China.
Also, in Apr 2017, Inovio has submitted a complete response to the FDA for the initiation of phase III study on VGX-3100 for HPV-related high grade cervical dysplasia, which was placed on clinical hold in Oct 2016. If the complete response is found to be satisfactory by the FDA to lift the clinical hold, Inovio will be able to start the phase III study in the second quarter of 2017.
Moreover, during the same period the company started a phase II study to evaluate the efficacy of VGX-3100 in women with HPV-related vulvar neoplasia. This is an indication with orphan status potential.
The enrolment of candidates has been completed for the phase I study of INO-1800, which is now being carried out independently by Inovio after Roche backed out. Data from the study is expected in the second half of 2017.
Inovio has started a phase I study on vaccines being developed for Ebola, Zika and Middle East Respiratory Syndrome (MERS). In Apr 2017, the company reported positive results from the study, which demonstrated robust immune responses with favorable safety profile.
During the quarter, Inovia entered into an immuno-oncology collaboration agreement with Regeneron Pharmaceuticals, Inc. (NASDAQ:REGN) . Under the agreement, Inovio will conduct a phase I/II study to evaluate the combination of its T-cell activating immunotherapy, INO-5401, with Regeneron’s PD-1 checkpoint inhibitor, REGN2810.
Zacks Rank & Key Picks
Inovio currently carries a Zacks Rank #4 (Sell). A better-ranked stock in the health care sector is VIVUS, Inc. (NASDAQ:VVUS) , which sports a Zacks Rank #1 (Strong Buy). You can see the complete list of today’s Zacks #1 Rank stocks here.
VIVUS’s loss per share estimates narrowed from 50 cents to 39 cents for 2017, over the last 30 days. The company posted positive earnings surprises in all of the four trailing quarters with an average beat of 233.69%.
The Best & Worst of Zacks
Today you are invited to download the full, up-to-the-minute list of 220 Zacks Rank #1 "Strong Buys" free of charge. From 1988 through 2015 this list has averaged a stellar gain of +25% per year. Plus, you may download 220 Zacks Rank #5 "Strong Sells." Even though this list holds many stocks that seem to be solid, it has historically performed 6X worse than the market. See these critical buys and sells free >>
Roche Holding AG (RHHBY): Free Stock Analysis Report
Regeneron Pharmaceuticals, Inc. (REGN): Free Stock Analysis Report
VIVUS, Inc. (VVUS): Free Stock Analysis Report
Inovio Pharmaceuticals, Inc. (INO): Free Stock Analysis Report
Original post
Zacks Investment Research