InMed Pharmaceuticals Inc (TO:IN) recently reported results for the second quarter of FY19 and is on track to bring INM-750 for epidermolysis bullosa (EB) into the clinic by the end of the year. The company recently completed two topical seven-day dose-ranging studies in pigs; the trials evaluated skin irritation, pharmacokinetics, histology and skin/drug concentrations and InMed is on the verge of selecting the contract manufacturing organization that will produce the topical cream necessary for the Phase I trial. Progress on biosynthesis continues as the company has initiated technology transfer activities to the National Research Council Canada (NRC), which will support fermentation scale-up.
INM-750 continues to progress through testing
InMed recently completed two topical seven-day dose-ranging studies that evaluated skin irritation, pharmacokinetics, histology and skin/drug concentrations. Importantly, the studies showed there were no drug-related adverse effects on the skin and systemic cannabinoid exposure was minimal after topical administration despite a dosing level 100- to 1,000-fold higher than the anticipated clinical dose.
CTA filing on track for H219
Given the positive results of the recent preclinical dose-ranging studies for INM-750 InMed continues to believe it is on track for a Clinical Trial Application (CTA) filing in H219 with initiation of the Phase I study by the end of the calendar year. The final selection of a contract manufacturing organization to produce drug product for the clinical trial is expected by the end of February and will help bring further confidence to expected timelines.
Biosynthesis development continuing
InMed initiated technology transfer activities to the NRC to support scale-up activities in late 2018. The company will soon begin the portion of the process that relates to the transfer of the gene construct containing the coding sequence for the enzymes responsible for cannabinoid production. Parameter optimization for fermentation scale-up activities will begin once this process is completed.
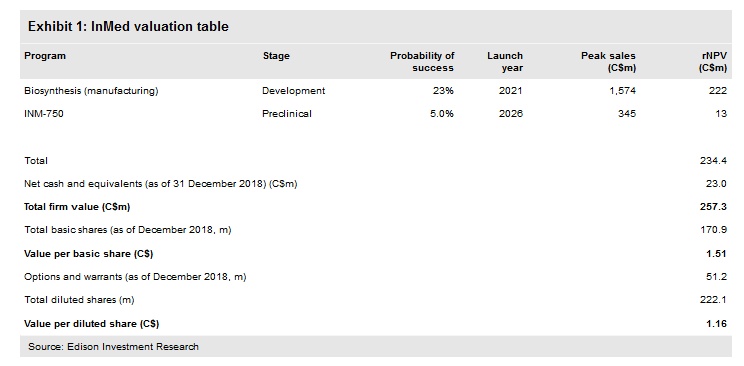
Valuation: C$257m or C$1.51 per basic share
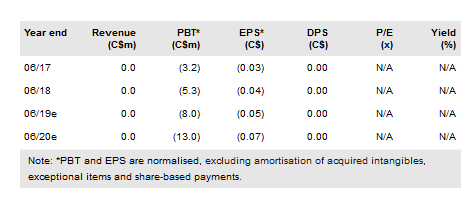
We have adjusted our valuation from C$232m or C$1.36 per basic share (C$1.05 per diluted share) to C$257m or C$1.51 per basic share (C$1.16 per diluted share) mainly due to rolling forward our estimates, although this was mitigated by lower net cash. InMed had C$23.0m in cash at 31 December and we believe this provides a runway through to FY20.
Business description
InMed Pharmaceuticals is a Canada-based biopharmaceutical company focused on manufacturing and developing cannabinoids. Its biosynthesis platform may be able to produce cannabinoids for less cost and with improved purity compared to currently used methods. The company is also developing a proprietary pipeline, including INM-750 for epidermolysis bullosa, a serious, debilitating orphan indication.
Q2 FY19 results and R&D update
InMed recently reported its Q2 FY19 results and provided a development update. The company continues to run a series of preclinical studies for INM-750 for the treatment of EB. InMed recently completed two topical seven-day dose-ranging studies, which evaluated skin irritation, pharmacokinetics, histology and skin/drug concentrations in pigs. Importantly, the studies showed there were no drug-related adverse effects on the skin and systemic cannabinoid exposure was minimal after topical administration despite a dosing level 100- to 1,000-fold higher than the anticipated clinical dose. InMed plans to initiate stability studies and other regulatory enabling studies prior to a CTA filing in H219 (specifically, the company is guiding for a filing in the fall).
The company expects to initiate the Phase I trial by the end of the calendar year. Currently the company expects to enrol approximately 30 patients in total for the trial and for it to be a two-part study that will test two different drug concentrations in each part. The first cohort will evaluate the safety, tolerability and PK of INM-750 cream once daily for 14 days in healthy volunteers with normal, intact skin. In a second cohort of healthy volunteers, the company will test the local safety and tolerability of applying INM-750 cream to small wounds once daily for seven days (the small wounds will be created at the clinical site and will largely mimic the types of wounds typically seen in EB simplex patients). All that said, the final design of the study may be influenced by regulatory feedback.
Importantly InMed has already selected a clinical research organization (CRO) to run the human clinical trials and is on the verge of selecting a contract manufacturing organization that will produce the topical cream necessary for the Phase I trial, which provides us with additional confidence that the current timelines will be met. With regards to the timing of the Phase I trial itself, it will likely enrol quickly (the company expects the enrolment period to be “a matter of weeks”) so results should be released sometime in the first half of 2020.
With regards to biosynthesis, InMed previously announced in October that it had entered into a development agreement with the National Research Council of Canada (NRC), the primary research and technology organization of the Canadian government, for biofermentation development and scale-up processes at the NRC’s dedicated facility in Montreal. Technology transfer activities to the NRC began in late 2018 and the company has already completed the transfer of the protocols for high-performance liquid chromatography. InMed will soon begin the portion of the process that relates to the transfer of the gene construct containing the coding sequence for the enzymes responsible for cannabinoid production. Parameter optimization for fermentation scale-up activities will begin once this process is completed. Additionally, the company signed a master service agreement with a contract development and manufacturing organization (CDMO) for downstream purification processing. The processes will be optimized in parallel at both the NRC and the CDMO and then later combined with larger-scale batches, expected by the end of the year. Commercial-scale batches are likely to occur in 2020.
Valuation
We have adjusted our valuation from C$232m or C$1.36 per basic share (C$1.05 per diluted share) to C$257m or C$1.51 per basic share (C$1.16 per diluted share) mainly due to rolling forward our NPVs, although this was mitigated by lower net cash.
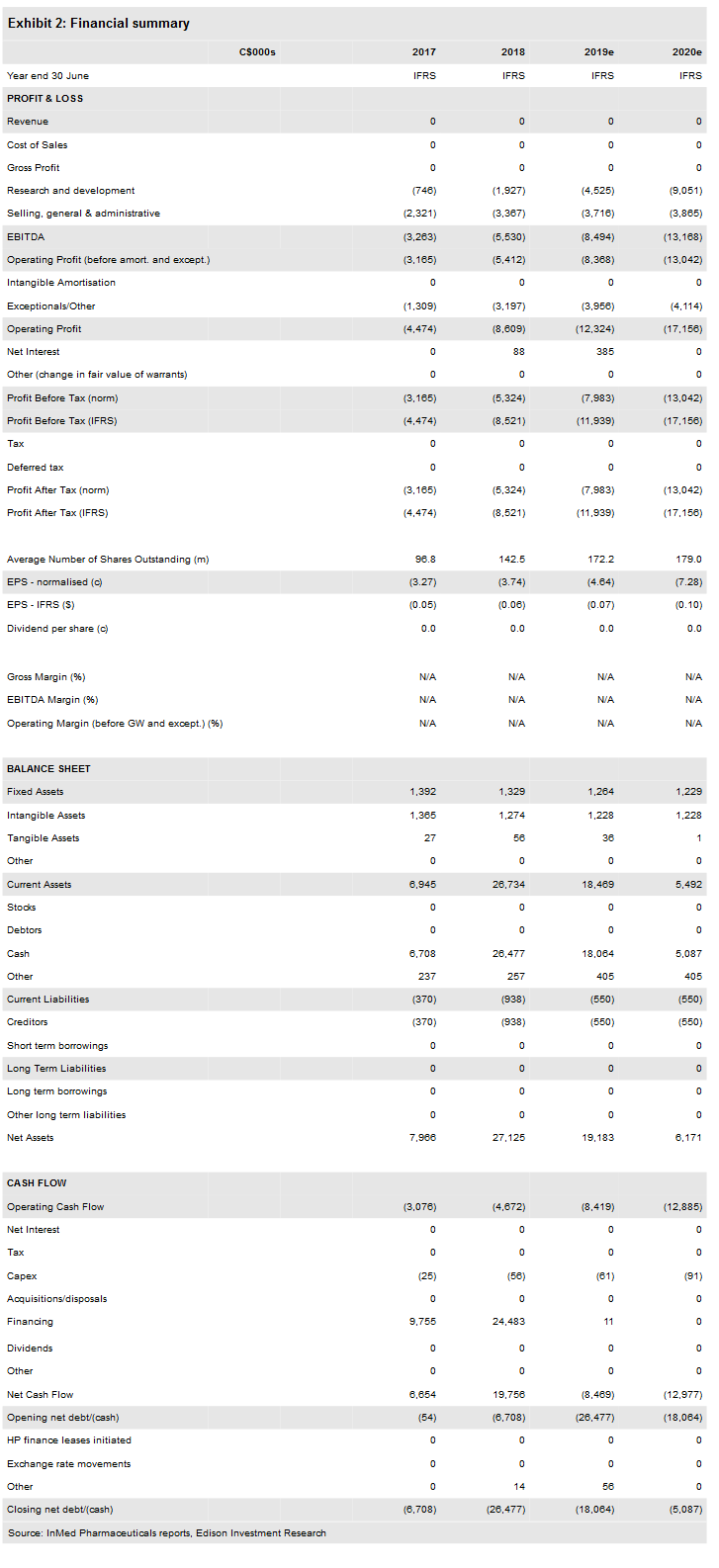
Financials
InMed reported an operating loss, excluding the gain from foreign exchange, of C$2.9m in Q2 of FY19 (the quarter ending 31 December 2018), up from C$1.5m for the same period a year ago. R&D expenses were C$0.9m in Q219, up from C$0.4m in the same quarter last year. We have increased our R&D estimates by C$0.7m in FY19 and by C$1.3m in FY20 as the spending was a bit higher than we had expected.
The company had C$23.0m in cash at 31 December and had an operating cash burn of C$3.6m through the first half of FY19. As we expect R&D expenditures to accelerate in the coming quarters, we believe its cash level provides a runway through to FY20.