Entering pivotal territory
GW Pharmaceuticals Plc (NASDAQ:GWPH) has initiated its first Phase III pivotal study for Epidiolex in Dravet syndrome, a highly debilitating and treatment refractory form of childhood epilepsy. This is the first of four planned Phase III trials with Epidiolex (one more in Dravet, two in Lennox-Gastaut syndrome), all due to begin in Q215. Epidiolex is a major valuation driver, so top-line data by end-2015 from the first Phase III now underway is a significant catalyst. We also look forward to further physician-led data to be presented at AAN in mid-April to highlight Epidiolex’s potential across a wide range of childhood epilepsies.
First Phase III up and running…
The pivotal trial now underway is the second part of a two-part (Phase II/III) randomized, double-blind, placebo-controlled study of Epidiolex to treat Dravet syndrome in children already treated with other anti-epileptic drugs. This part B will recruit up to 100 new patients, dosed at 20mg/kg/day for 14 weeks, and the primary efficacy endpoint is the percentage change from baseline in convulsive seizure frequency over the treatment period. The dose was selected after a DSMB review of safety and PK data from part A, involving 34 patients treated for three weeks.
…to be followed by three more…
GW expects to soon initiate the second Phase III trial (n=120) in DS, and to start two Phase III studies (n=80 + n=120) in Lennox-Gastaut syndrome (LGS) in early Q215. Recruitment into all these further trials is expected to complete in 2015, with results in H116, leading to a potential NDA for Epidiolex in mid-2016.
…while AAN data may point to a wider opportunity
At the American Academy of Neurology (AAN) meeting (18-25 April), further data are expected from the US Expanded Access Program, whereby Epidiolex is being used to treat a range of childhood epilepsies. To date, 235 children have been treated at 13 clinical sites and GW presented highly encouraging data (36% reduction in median seizure frequency) from 58 patients in October 2014. AAN should see updated data from ~100 patients and fresh insight into other epilepsies.
Valuation: $1.78bn ($90.20/ADR) ahead of catalysts
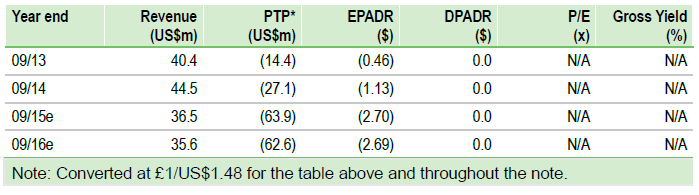
Our DCF valuation has increased slightly to $1.78bn or $90.20/ADR (vs $1.75bn, $88.51/ADR), on rolling our model forward to H115 ($207m estimated cash at 31 March 2015) and currency benefit (£/$1.48 vs 1.52). We note this represents our fair value ahead of key catalysts in 2015, namely the AAN data and first Phase III data, which could result in widening the target patient population (beyond DS/LGS) and greater probabilities of success for Epidiolex (currently 50%).
To Read the Entire Report Please Click on the pdf File Below