Formycon AG (DE:FYB) partner Bioeq IP (Bioeq) plans to file FYB201, a Lucentis biosimilar candidate, to treat neovascular age-related macular degeneration (nAMD) with the FDA imminently in Q419. The US launch could be in 2021 and the EU in 2022. H119 revenues were €17.2m from partners for product development services. There are three main projects. Bioeq is the partner on FYB201, Santo on FYB203 (an Eylea biosimilar candidate) and a joint venture with Aristo Pharma on FYB202 (a Stelara biosimilar candidate). FYB202 is due to enter a Phase I trial soon. Formycon guides for FY19 revenues of about €35m, formerly €34m. End-June cash was €7.5m, effectively €19.8m including the final €12.3m of proceeds from the €17.3m private placing in March 2019.
FYB201 and FYB203 target the major nAMD markets
Formycon has two biosimilar projects targeting the nAMD market. Based on positive 2018 data, the global exclusive partner, Bioeq, plans to make regulatory filings in Q419 in the US and in the EU in Q120. A deal with a US marketing partner is expected to be agreed to enable a launch in 2021. The EU launch is possible in 2022. Global 2018 Lucentis sales rose 9% to $3.7bn. Formycon’s preclinical Eylea biosimilar candidate, FYB203, also for nAMD, is licensed to Santo in a deal worth over €100m according to management. Global sales of Eylea were $6.7bn in 2018; core patents expire in 2023 (US) and 2025 (EU). Formycon assumes strong biosimilar demand due to healthcare cost pressures.
A stellar opportunity through a JV
Formycon has made significant progress on FYB202 (a Stelara biosimilar for Crohn’s disease, psoriasis and (EU only) ulcerative colitis) through a joint venture with Aristo Pharma; Formycon owns 24.9%. Stelara (2018 sales $5.2bn) has a different mode of action to anti-TNF agents (lead product Humira, $19.9bn in 2018) so should be protected from the fierce competition developing in the anti-TNF area. The project is now guided to start imminently in Q419, formerly mid-2019. Formycon needs to fund its share of the costs, €4.7m in H1, but shares the profits so this could be very lucrative. Stelara patents expire in 2023 (US) and 2024 (EU).
Valuation: yet to reflect likely progress from Q4 2019
Formycon’s market cap is about €310m with an effective EV of €290m. In Q4, starting the regulatory review of FYB201, initiating clinical development of FYB202 and signing a US FYB201 marketing partner should add value. Including cash, unpaid placing proceeds and trade receivables, June liquid assets were €27.8m.
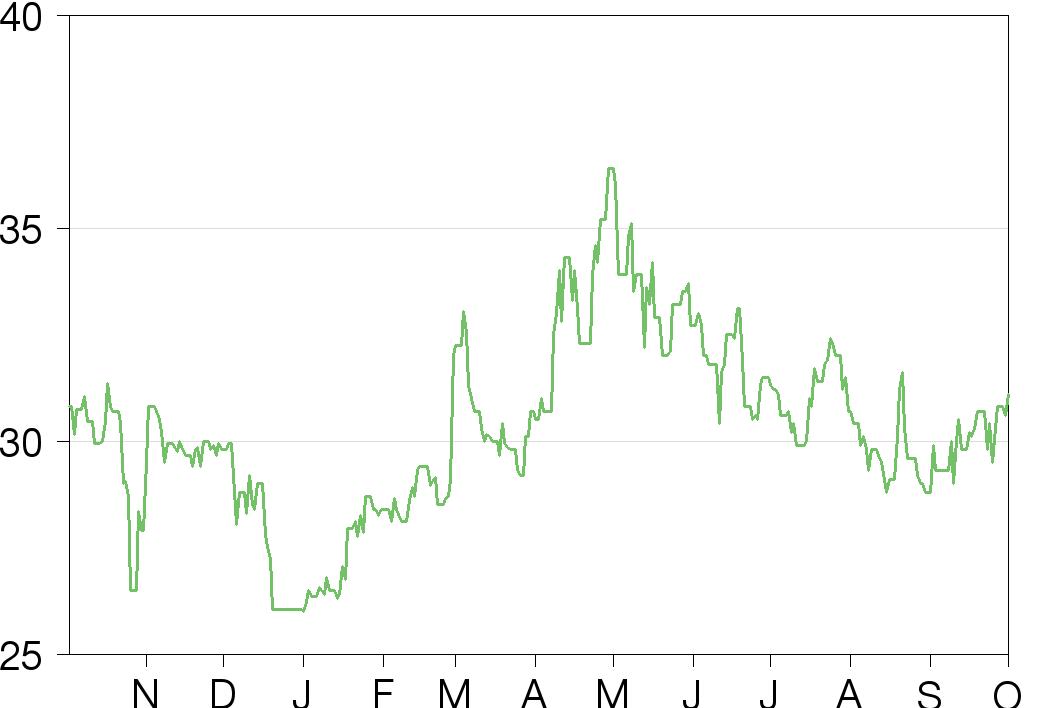
Consensus estimates
Share price graph
Business description
Formycon is a biotechnology company developing biosimilars. The main lead is FYB201, a Lucentis biosimilar candidate that has completed Phase III and is entering regulatory review. FYB203 is an Eylea candidate biosimilar in preclinical. They are both out-licensed. FYB202, a biosimilar candidate of Stelara, is being developed in a joint venture
Financials: H119 results review
Formycon reported H1 revenues of €17.2m as research and development payments from partners vs €24.6m in H118. The operating loss was €0.7m (vs €8m in H118, including non-recurring items).
The FYB202 project is run through a joint venture company (FYB202 GmbH & Co KG) with Aristo Pharma, which requires periodic investment depending on its undisclosed financial position. According to Formycon, the projected development cost is estimated at about €130m, which implies a total contribution by Formycon of €32.4m. There was an H1 investment of €4.7m in the joint venture which added to the €15.97m invested in H118 to give a balance sheet asset of €20.7m. Further investment of €12m may be required at some point.
Formycon made a €17.26m placing on 22 March 2019 with Swiss investor M&H Equity (M&H). The investment was at €29.90 per share for the 577,397 shares. However, M&H only part paid the shares to a value of €5m. To resolve the matter on June 26 2019, Wendeln & Cie. KG, an asset management company controlled by the shareholder and Supervisory Board member Peter Wendeln, assumed all rights from the subscription by M&H and acquired the 577,397 shares. This increased the combined shareholding of Peter Wendeln and companies affiliated with him from about 18.9% to about 24.6%. In the 30 June balance sheet, there remained €12.3m as a creditor.
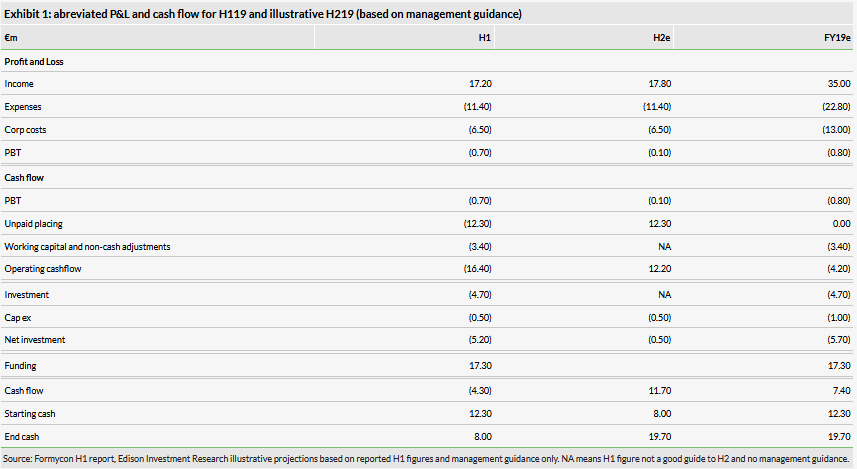
The H119 profit and loss and cash situation is summarised in Exhibit 1 with a simple projection for H2 based on Edison estimates and management guidance.
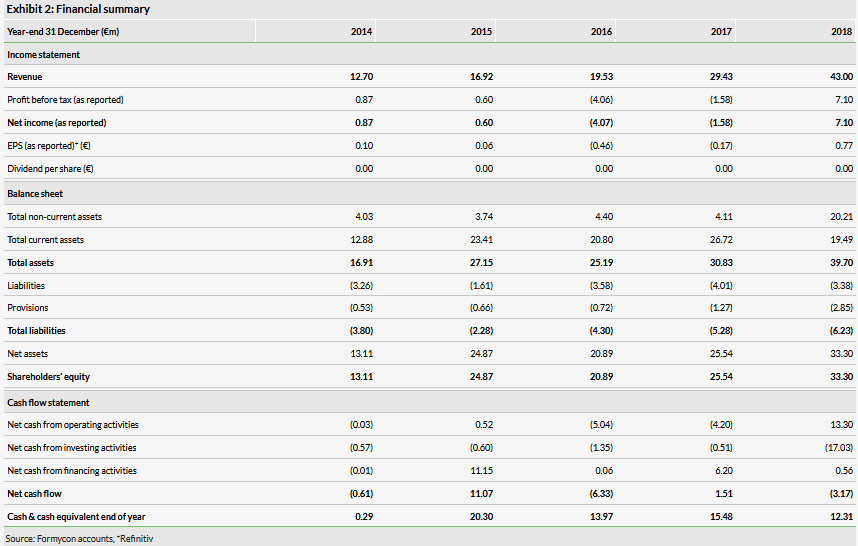
After the €17.3m cash raised, less the creditor of €12.3m, cash and cash equivalents was €7.5m, down from €12.3m as of 31 December 2018. Formycon expects H219 revenues of about €18m. Formycon has guided to a small overall reported loss for FY19. If H219 has similar expenses to H1, about €18m, year-end cash after settlement of the €12.3m creditor might be about €19m, but this will vary depending on underlying development costs and unknown working capital movement and H2 JV investment. The historic financial position is shown in Exhibit 2.
A marketing partner for FYB201 in the US might be agreed, according to Formycon, in Q419. This will allow a 2021 US launch assuming an FDA approval. In Europe, regulatory review is expected from Q120 with potential launch in 2022 on European patent expiry.
Three key projects for global markets
The lead project is FYB201, a biosimilar candidate to Lucentis now entering regulatory review. The project on the Stelara biosimilar candidate has completed a pilot phase and is due to enter Phase I soon in Q419 and the Eylea biosimilar candidate, FYB203, is preclinical; Formycon estimates that it may enter clinical development in mid-2020. A further project is currently undisclosed.
Although we cite the reference product sales for each project, the in-market biosimilar price will be typically lower by c 15–20% initially and possibly 30–50% if competition is tough. One element in these markets is that switching rates from established reference products to slightly cheaper, but not necessarily identical biosimilars has often been slow. For approval, a biosimilar must show comparable safety, efficacy and immunogenicity to the original ‘reference’ products so, technically, there is no reason for prescribers not to switch. In May 2019 the FDA set out guidelines to establish full interchangeability with reference products. This might enhance US uptake of biosimilars.
A new set of EU rules were approved on 20 May 2019 and allow potential competitors to manufacture biosimilars in Europe from six months before the patent and any supplementary protection expires. Formycon does not expect this, in practice, to accelerate the launch of FYB201, but it may enable more rapid launches for other products, for example FYB202.
FYB201: viewing a competitive space
Lucentis (ranibizumab, Roche (US) Novartis (EU)) had 2018 sales of $3.7bn. Ranibizumab is a humanised monoclonal antibody fragment produced in Escherichia coli cells by recombinant DNA technology; it binds vascular endothelial growth factor-A (VEGF-A). The later competitor product Eylea (Regeneron) initially took sales and market growth from Lucentis. The position has stabilised and Lucentis sales grew 9% in 2018. Lucentis patents expire in 2020 in the US and 2022 in Europe.
Formycon licensed FYB201 to Bioeq, based in Zürich. Bioeq comprises a joint venture between Polpharma (a Polish pharmaceutical company) and the Strüngmann family’s investment company. The clinical studies for FYB201 are run by a Bioeq subsidiary, Bioeq GmbH, based in Germany. A US marketing partner is expected to be agreed, according to Formycon, in Q4 2019.
The Phase III study, COLUMBUS-AMD (NCT02611778), reported its primary endpoint in May 2018, which confirmed comparable efficacy between FYB201 and Lucentis in patients with nAMD (included in wet AMD). The clinical primary endpoint measured the change in the best corrected visual acuity after eight weeks. All secondary endpoints were also met. The Phase III is now completed with no observed issues about the safety and immunogenicity of FYB201.
Competitors include:
Samsung (KS:005930) Bioepis (Korea, sales via Biogen (NASDAQ:BIIB)) with a Phase III (NCT03150589) completing in Q419; the primary endpoint may have completed in January with the safety phase ongoing.
Xbrane (Sweden) is partnered with STADA, a privately-owned German-based generics and OTC company that sells (some) in-licensed biosimilars. The global Phase III (NCT03805100, XPLORE) has a primary endpoint due in May 2020. Xbrane management expect sales from Q122; STADA currently has no US distribution at all and selective EU and Asian coverage.
Roche has a novel antibody, faricimab, in a Phase III (primary data Q321, completion Q422. The excellent Phase II performance was based on one injection every 16 weeks; this might compete in the premium market after 2023. Roche is also trialling a Lucentis medical device delivery system to protect its core franchise; the primary endpoint will be reached in Q1 2021.
Novartis has filed brolucizumab, a scFv antibody fragment against VEGF-A, with the FDA. This has a priority review. If approved by the FDA, in mid-October, Novartis anticipates launching brolucizumab by the end of 2019. It claims a dosing interval of up to every 12 weeks.
A market complication will be biosimilar versions of Avastin. Avastin has the same mode of action as Lucentis but is a large monoclonal rather than a smaller fragment. Avastin is used off label for nAMD as it is cheaper; generic versions could accentuate this gap. This use is believed to be decreasing because the larger Avastin pack size gives handling and sterility issues.
FYB202: A stellar project
Formycon is developing a biosimilar, FYB202, to Stelara (ustekinumab, Jansen). The product is an antibody that binds interleukin-12 (IL-12) and IL-23. These potent cytokines drive the immune response so neutralising them controls autoimmune diseases such as Crohn’s disease and psoriasis. In Europe, it was EMA approved in July 2019 for moderate to severe active ulcerative colitis that was unresponsive to other therapies. It is not used for rheumatoid arthritis (a massive market) but is effective for psoriatic arthritis (Veale and Fearon, 2015) . Stelara has its main US patent expiry in September 2023 in the US and in January 2024 in Europe. Sales were $5.2bn in 2018 driven by the 2017 approval in Crohn’s disease.
The general market for these anti-inflammatory therapies targeting cytokines such as IL-12 and tumour necrosis factor (TNF) is likely to be competitive with the patent expiries of huge ($19.9bn) global franchises such as Humira (adalimumab, an anti-TNF monoclonal), mainly used for rheumatoid arthritis, but also Crohn’s disease and psoriatic arthritis. Stelara has a different mode of action to anti-TNF therapies as it binds a different set of inflammatory messenger proteins. This different approach might be better for some patients.
In Europe, the Humira patent expired in October 2018 and four Humira biosimilars are sold: Amgevita (Amgen (NASDAQ:AMGN)), Hyrimoz (Sandoz), Hulio (Mylan/Fujifilm Kyowa Kirin Biologics) and Imraldi (Biogen/Samsung Bioepis). International (mainly European) 2018 Humira sales were $6.3bn after a 14.8% Q4 decline due to European patent expiry.
In the US, 2018 sales were $13.7bn, up 9%. The primary patent expired in 2016 and three biosimilars are already approved: Amjevita (adalimumab-atto, Amgen (NASDAQ:AMGN)), Hyrimoz (adalimumab-adaz, Sandoz) and Cyltezo (adalimumab-adbm, Boehringer Ingelheim), with Imraldi (Biogen/Samsung Bioepis) under FDA review. However, so far patents and agreements with Amgen mean that these biosimilar launches will be delayed and then phased over 2023.
FYB202 has been licensed to privately owned Aristo Pharma through a joint venture vehicle, FYB202 GmbH & Co. KG. Formycon holds 24.9% and will therefore have to fund this proportion of the clinical and development costs, amount undisclosed, but will receive that share of profits. The pilot phase of the project has ended. After FDA meetings, Phase I could now start in early Q4 2019. This implies Phase III starting in 2020-21. There are no clinical trials disclosed. Others such as Icelandic company Alvotech and Australian NeuClone are in preclinical development of Stelara candidate biosimilars; no trials are listed.
FYB203: An eye on the future
FYB203 is a preclinical project to develop an Eylea biosimilar. Regeneron had 2018 Eylea sales of $6.7bn (split $4bn US and $2.7bn international). Eylea is used in a similar way to Lucentis but has a different mode of action as it binds both VEGF-A and placental growth factor; Lucentis binds only VEGF-A. The Eylea maintenance dose interval is every eight weeks, double that of Lucentis although Formycon notes that in reality clinical surveys show that the use patterns are similar. US Eylea patents start to expire in 2020 but there seem to be patent extensions (Sharma et al., 2018) that will prevent biosimilar competition in the US until 2023. European patents expire in 2025. In addition, Eylea formulation patents do not expire until 2027–28. Formycon has filed patents for an alternative formulation that has shown preclinical intraocular bioequivalence.
Formycon has a global licensing deal with Santo Holding and gains sales-related royalties. Preclinical work is underway; a Phase I/III trial might start in 2020, according to management.
Competitors include a joint development programme between Momenta Pharmaceuticals and Mylan (NASDAQ:MYL) NV on the Eylea biosimilar, M710. A Phase III trial (NCT03610646) in 324 patients is underway and due to report primary data in Q120. Alteogen (South Korea) has a preclinical project: ALT-L9, so it might be a potential future competitor.
Valuation: Clear pipeline and solid financial position
So far, the biosimilar business has been less aggressive than small molecule generics. This is because the technology is complex and the development and production costs of biologicals are high, which sustains margins. Formycon expects switch rates to increase due to cost pressures on healthcare systems.
Formycon’s market cap is about €310m with about €1m of debt liabilities. Adding liabilities and subtracting €19.8m effective cash gives an EV of about €290m. Value progression depends on the likely royalties from FYB201 and FYB203 and the 24.9% profit share from the joint venture on FYB202. Filing for FDA and EMA review and signing a US marketing partner are anticipated short-term value points.
Formycon is in a strong position on Lucentis biosimilar development with a completed, positive Phase III and planned early Q419 BLA application to the FDA with EMA submission in Q120. However, Samsung (KS:005930) Bioepis is not far behind in the US and both Samsung Bioepis and Xbrane might be ready for the European patent expiry in 2022, making the market competitive.
The other projects are further behind, but FYB202 could develop quickly through the JV. Formycon’s 24.9% share of costs will need funding; this is worthwhile as it gives an increased profit share. The Stelara franchise appears not to be targeted by many developers but the related Humira EU market is a battleground, with the US protected until 2023 and competitors queuing to pile in. Stelara might be a more protected market as not all Crohn’s and psoriasis patients will use anti-TNF products, which might protect volume and margins. The recent EU ulcerative colitis indication extension should boost the potential market.
It is harder to assess FYB203. An Eylea biosimilar should sell very well but competitors seem, oddly, very limited to date given high sales and relatively close US patent expiry in mid-2023. The Momenta Pharmaceuticals and Mylan (NASDAQ:MYL) joint venture is the clear leader so far. We would expect further entrants over the next few years. The market will also be complicated by new, long-acting antibody fragment products from Novartis and Roche at premium prices. However, while the new products may be more convenient, if approved, neither of them has shown clinical superiority over Lucentis and Eylea so the biosimilar market will be substantial and very valuable.
Investment case summary
Formycon has a robust financial position with high revenues, cash for investment and a strong product pipeline targeting major global markets. It can now develop its own proprietary pipeline, which should add further value.
Although there are always technical risks and delays in development, these are manageable, more predictable and much lower than in mainstream therapeutic development. The main uncertainties are on exact product launch dates, competition and the ability of partners to market effectively in large, complex global markets. Assuming these aspects are resolved, Formycon should become one of the leading and very profitable biosimilar companies.