Exelixis, Inc.’s (NASDAQ:EXEL) European partner Ipsen announced that the European Commission (EC) has granted approval to Cabometyx (cabozantinib) for an expanded indication. The EC approved Cabometyx 20 mg, 40 mg and 60 mg for the first-line treatment of adults with intermediate- or poor-risk advanced renal cell carcinoma (RCC) — a kind of kidney cancer in the European Union.
The approval was expected in March. The company received positive opinion from the Committee for Medicinal Products for Human Use (“CHMP”) for the use of Cabometyx for the above indication.
The EU approval in the first-line setting was based on results of the CABOSUN trial, which met its primary endpoint of improving progression-free survival compared with Pfizer’s (NYSE:PFE) Sutent (sunitinib) in patients with previously untreated advanced RCC.
Cabometyxis already marketed in the EU for advanced RCC in adults following prior vascular endothelial growth factor (VEGF)-targeted therapy. In the United States, Cabometyx was approved for first-line treatment of advanced RCC in December last year, approximately two months ahead of the assigned Prescription Drug User Fee Act action date.
Cabometyx generated $128.9 million net product revenues in the first quarter of 2018 driven by growth in demand following U.S. approval in the first lien setting. An approval in Europe will further boost Exelixis’ top line.
Per the terms of the collaboration and license agreement with Ipsen, Exelixis will receive a milestone payment of $50 million for the EC approval, of which about $46 million was recognized as collaboration revenues in the first quarter of 2018. The payment will be made by Ipsen within the next 70 days.
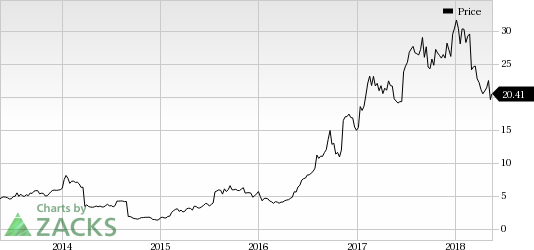
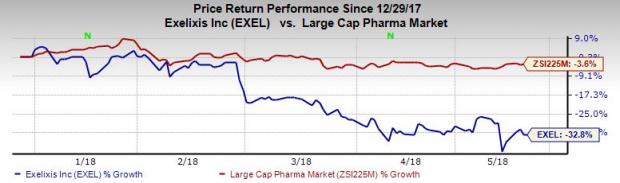
Exelixis’ shares have lost 32.8% over a year, wider than the industry’s decline of 3.6%.
We remind investors that Exelixis entered into a collaboration and license agreement with Ipsen in February 2016 for the commercialization and further development of cabozantinib whereby Ipsen received exclusive commercialization rights for current and potential future cabozantinib indications outside the United States, Canada and Japan.
Exelixis has also inked agreements with Bristol-Myers (NYSE:BMY) and Roche Holding (OTC:RHHBY) to develop cabozantinib in combination with their immunotherapy agents in 2017.
Zacks Rank
Exelixis has a Zacks Rank #3 (Hold).You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Exelixis, Inc. Price
Will You Make a Fortune on the Shift to Electric Cars?
Here's another stock idea to consider. Much like petroleum 150 years ago, lithium power may soon shake the world, creating millionaires and reshaping geo-politics. Soon electric vehicles (EVs) may be cheaper than gas guzzlers. Some are already reaching 265 miles on a single charge.
With battery prices plummeting and charging stations set to multiply, one company stands out as the #1 stock to buy according to Zacks research.
It's not the one you think.
Roche Holding AG (RHHBY): Free Stock Analysis Report
Bristol-Myers Squibb Company (BMY): Free Stock Analysis Report
Pfizer Inc. (PFE): Free Stock Analysis Report
Exelixis, Inc. (EXEL): Free Stock Analysis Report
Original post