Graspa safety reinforced by EAP
A recent update from the Graspa Expanded Access Program (EAP) in France reaffirms its impressive safety profile, with patients allergic to Lasparaginase able to tolerate multiple Graspa doses (red blood cell encapsulated L-asparaginase). This is one of Graspa’s key benefits, which should allow uptake in leukaemia patients who cannot be treated with current forms of L-asparaginase, a mainstay therapy. Graspa EU filing is on-track for mid-2015 and Phase III data will be presented at upcoming conferences, helping to raise physician awareness.
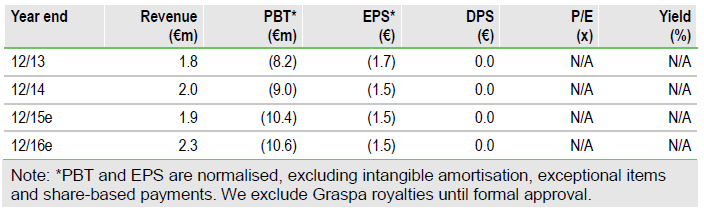
Further confirmation of Graspa’s safety with EAP
An Expanded Access Program is ongoing in France for patients who cannot tolerate L-asparaginase (either derived from E. coli or Erwinia chrysanthemi). To date 12 patients have been enrolled in the trial with a DSMB safety review in seven, who had received on average 3.4 doses of Graspa (range 2-7 doses). The DSMB concluded that the trial should continue to enrol patients as planned. Given these are ALL (acute lymphoblastic leukaemia) patients with allergy to L-asparaginase, the ability to tolerate multiple Graspa doses reinforces its safety profile.
Additional Phase III Graspa data at ASCO and EHA
The positive Graspa Phase III ALL trial will be presented at both ASCO and EHA conferences, helping to raise awareness amongst physicians ahead of launch. In particular, mature complete remission (CR) data will be presented, demonstrating 65% CR on Graspa compared to 39% on L-asparaginase (p=0.026).
EU filing by mid-2015; multiple other Graspa events
Erytech Pharma (PARIS:ERYP) anticipates Graspa ALL filing in Europe will be complete around mid-2015. A Phase II AML (acute myeloid leukaemia) trial in Europe is also ongoing, funded by partner Recordati, with data expected in 2016; the trial has already successfully completed two DSMB’s. In the US, a confirmatory safety study in ALL is ongoing; Erytech is seeking to accelerate US development in both ALL and AML, which is being discussed with the FDA. A Phase II pancreatic cancer is ongoing with the first DSMB anticipated around mid-2015. Erytech is also planning to start a Phase II NHL (non-Hodgkin’s lymphoma) trial in H215.
To Read the Entire Report Please Click on the pdf File Below