This year could be transformative for CytRx (CYTR) as it delivers important clinical datapoints, regulatory milestones and Phase III starts for its cancer pipeline. Major value inflection points include FDA agreement on the aldoxorubicin pivotal study in second-line soft tissue sarcoma (STS), aldoxorubicin Phase II data in first-line STS and tamibarotene Phase II results in advanced lung cancer. We value the company at $120m, or $4.00 per share, which could rise to $180m, or $5.80 per share, following positive clinical data.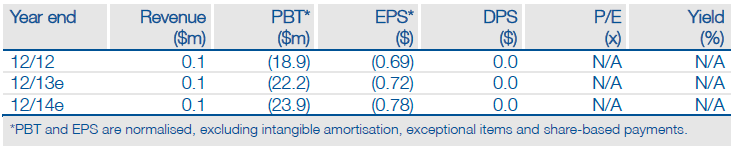
Multiple clinical catalysts in 2013
CytRx’s recent FY12 report outlined a rich newsflow profile for 2013, including four clinical study readouts - three aldoxorubicin, one tamibarotene - and start of the aldoxorubicin pivotal Phase III trial in second-line STS. The major value inflection points, in our view are: (1) FDA agreement (SPA grant) for the second-line Phase III trial, (2) aldoxorubicin Phase II data in first-line STS, and (3) tamibarotene Phase II results in advanced non-small cell lung cancer (NSCLC). Our updated financial model projects cash of $16.1m at year-end 2013, which provides a runway into late 2014.
Aldoxorubicin: Targeting Phase III start in Q313
We anticipate an SPA for the second-line STS Phase III study in Q213 and first patient enrolment in Q313. Separately, results of two supportive Phase Ib trials in advanced solid tumours are due Q213. In H213, we expect topline results from the Phase IIb study vs doxorubicin in first-line STS; PFS and safety data favouring aldoxorubicin would increase our confidence in the second-line opportunity and could drive a re-rating in CytRx’s valuation. Finally, competitor Phase III readouts (Ziopharm’s palifosfamide, Threshold’s TH-302) in H113 could clarify the competitive landscape in first-line STS.
Tamibarotene: Phase II lung cancer data in Q413
In H213, we anticipate headline results from tamibarotene Phase IIb study in advanced non-small cell lung cancer (NSCLC). The global trial is evaluating chemotherapy (paclitaxel, carboplatin) +/- tamibarotene as front-line therapy in 140 patients with stage IIIB or IV disease. The study recently completed enrolment, which points to progression free survival (PFS) data sometime in Q413.
Valuation: rNPV of $120m
We value CytRx at $120m, or $4.00/share, based on a NPV analysis. This includes aldoxorubicin in second-line STS ($70m), aldoxorubicin in second-line pancreatic cancer ($34m), tamibarotene in front-line NSCLC ($44m), year-end 2013 cash ($16m) and operating costs ($-43m).
To Read the Entire Report Please Click on the pdf File Below.
- English (UK)
- English (India)
- English (Canada)
- English (Australia)
- English (South Africa)
- English (Philippines)
- English (Nigeria)
- Deutsch
- Español (España)
- Español (México)
- Français
- Italiano
- Nederlands
- Português (Portugal)
- Polski
- Português (Brasil)
- Русский
- Türkçe
- العربية
- Ελληνικά
- Svenska
- Suomi
- עברית
- 日本語
- 한국어
- 简体中文
- 繁體中文
- Bahasa Indonesia
- Bahasa Melayu
- ไทย
- Tiếng Việt
- हिंदी
CytRx: Multiple Clinical Catalysts In 2013
Published 03/18/2013, 01:03 AM
Updated 07/09/2023, 06:31 AM
CytRx: Multiple Clinical Catalysts In 2013
2013 catalysts in view
3rd party Ad. Not an offer or recommendation by Investing.com. See disclosure here or
remove ads
.
Latest comments
Install Our App
Risk Disclosure: Trading in financial instruments and/or cryptocurrencies involves high risks including the risk of losing some, or all, of your investment amount, and may not be suitable for all investors. Prices of cryptocurrencies are extremely volatile and may be affected by external factors such as financial, regulatory or political events. Trading on margin increases the financial risks.
Before deciding to trade in financial instrument or cryptocurrencies you should be fully informed of the risks and costs associated with trading the financial markets, carefully consider your investment objectives, level of experience, and risk appetite, and seek professional advice where needed.
Fusion Media would like to remind you that the data contained in this website is not necessarily real-time nor accurate. The data and prices on the website are not necessarily provided by any market or exchange, but may be provided by market makers, and so prices may not be accurate and may differ from the actual price at any given market, meaning prices are indicative and not appropriate for trading purposes. Fusion Media and any provider of the data contained in this website will not accept liability for any loss or damage as a result of your trading, or your reliance on the information contained within this website.
It is prohibited to use, store, reproduce, display, modify, transmit or distribute the data contained in this website without the explicit prior written permission of Fusion Media and/or the data provider. All intellectual property rights are reserved by the providers and/or the exchange providing the data contained in this website.
Fusion Media may be compensated by the advertisers that appear on the website, based on your interaction with the advertisements or advertisers.
Before deciding to trade in financial instrument or cryptocurrencies you should be fully informed of the risks and costs associated with trading the financial markets, carefully consider your investment objectives, level of experience, and risk appetite, and seek professional advice where needed.
Fusion Media would like to remind you that the data contained in this website is not necessarily real-time nor accurate. The data and prices on the website are not necessarily provided by any market or exchange, but may be provided by market makers, and so prices may not be accurate and may differ from the actual price at any given market, meaning prices are indicative and not appropriate for trading purposes. Fusion Media and any provider of the data contained in this website will not accept liability for any loss or damage as a result of your trading, or your reliance on the information contained within this website.
It is prohibited to use, store, reproduce, display, modify, transmit or distribute the data contained in this website without the explicit prior written permission of Fusion Media and/or the data provider. All intellectual property rights are reserved by the providers and/or the exchange providing the data contained in this website.
Fusion Media may be compensated by the advertisers that appear on the website, based on your interaction with the advertisements or advertisers.
© 2007-2024 - Fusion Media Limited. All Rights Reserved.