Novartis (NVS) has returned NIC002, a smoking cessation vaccine, to Cytos Biotechnology (CYTN) but Pfizer (PFE) has started a Phase I study with VLP-IgE. The investment case is barely altered by these changes and still largely hinges solely on how its main asset, CYT003, progresses through a key Phase IIb trial in allergic asthma. The study is due to report early data in H114, with full results by end-2014. A positive outcome should mean that debt, due for repayment in February 2015, will be converted or repaid without difficulty, and that Cytos will have sufficient time to out-license CYT003.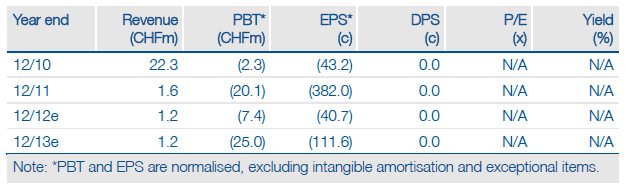
Novartis returns rights for NIC002...
Novartis has terminated development of NIC002, a therapeutic vaccine candidate for the treatment of nicotine addiction. This follows from a disappointing Phase II study, submitted in October 2009, which failed to meet the primary endpoint of increased smoking cessation. The other Novartis collaboration involves CAD106 for Alzheimer's disease and is in Phase II development.
... but Pfizer moves IgE programme into Phase I
Pfizer has started a Phase I trial with the anti-IgE vaccine with two different adjuvants in patients with perennial allergic rhinitis. The vaccine is based on Cytos’s virus-like-particle (VLP) platform. The 189-patient trial is expected to be completed by the end of 2014. Novartis and Roche sell the anti-IgE antibody omalizumab (Xolair) for the treatment of allergic asthma.
Key CYT003 trial to report early data in H114
The investment case for Cytos Biotechnology is still largely dependent on how its main asset, CYT003, progresses through a critical Phase IIb trial in allergic asthma. Full data from this study is expected by end-2014; if the trial is positive, we believe that current financing structures should mean that debt will be converted or repaid without difficulty, and that Cytos will have sufficient time to out-license.
Valuation: Upside dependent on CYTOO3 trial success
Our valuation falls by CHF4m to CHF94m or CHF4.19 per share following the removal of NIC002 and addition of VLP-IgE on our model. If CYT003 (currently worth CHF75m) is successful in the key Phase IIb trial, the value per share would rise to between CHF5.31 and CHF5.92 per share at FY14, assuming that the conversion of loan notes and exercising of outstanding warrants occurs as expected.
To Read the Entire Report Please Click on the pdf File Below.
- English (UK)
- English (India)
- English (Canada)
- English (Australia)
- English (South Africa)
- English (Philippines)
- English (Nigeria)
- Deutsch
- Español (España)
- Español (México)
- Français
- Italiano
- Nederlands
- Português (Portugal)
- Polski
- Português (Brasil)
- Русский
- Türkçe
- العربية
- Ελληνικά
- Svenska
- Suomi
- עברית
- 日本語
- 한국어
- 简体中文
- 繁體中文
- Bahasa Indonesia
- Bahasa Melayu
- ไทย
- Tiếng Việt
- हिंदी
Cytos Biotechnology - Musical Chairs
Published 02/11/2013, 06:46 AM
Updated 07/09/2023, 06:31 AM
Cytos Biotechnology - Musical Chairs
Musical chairs
3rd party Ad. Not an offer or recommendation by Investing.com. See disclosure here or
remove ads
.
Latest comments
Install Our App
Risk Disclosure: Trading in financial instruments and/or cryptocurrencies involves high risks including the risk of losing some, or all, of your investment amount, and may not be suitable for all investors. Prices of cryptocurrencies are extremely volatile and may be affected by external factors such as financial, regulatory or political events. Trading on margin increases the financial risks.
Before deciding to trade in financial instrument or cryptocurrencies you should be fully informed of the risks and costs associated with trading the financial markets, carefully consider your investment objectives, level of experience, and risk appetite, and seek professional advice where needed.
Fusion Media would like to remind you that the data contained in this website is not necessarily real-time nor accurate. The data and prices on the website are not necessarily provided by any market or exchange, but may be provided by market makers, and so prices may not be accurate and may differ from the actual price at any given market, meaning prices are indicative and not appropriate for trading purposes. Fusion Media and any provider of the data contained in this website will not accept liability for any loss or damage as a result of your trading, or your reliance on the information contained within this website.
It is prohibited to use, store, reproduce, display, modify, transmit or distribute the data contained in this website without the explicit prior written permission of Fusion Media and/or the data provider. All intellectual property rights are reserved by the providers and/or the exchange providing the data contained in this website.
Fusion Media may be compensated by the advertisers that appear on the website, based on your interaction with the advertisements or advertisers.
Before deciding to trade in financial instrument or cryptocurrencies you should be fully informed of the risks and costs associated with trading the financial markets, carefully consider your investment objectives, level of experience, and risk appetite, and seek professional advice where needed.
Fusion Media would like to remind you that the data contained in this website is not necessarily real-time nor accurate. The data and prices on the website are not necessarily provided by any market or exchange, but may be provided by market makers, and so prices may not be accurate and may differ from the actual price at any given market, meaning prices are indicative and not appropriate for trading purposes. Fusion Media and any provider of the data contained in this website will not accept liability for any loss or damage as a result of your trading, or your reliance on the information contained within this website.
It is prohibited to use, store, reproduce, display, modify, transmit or distribute the data contained in this website without the explicit prior written permission of Fusion Media and/or the data provider. All intellectual property rights are reserved by the providers and/or the exchange providing the data contained in this website.
Fusion Media may be compensated by the advertisers that appear on the website, based on your interaction with the advertisements or advertisers.
© 2007-2024 - Fusion Media Limited. All Rights Reserved.