Clavis Pharma’s investment case is entirely dependent on the late March outcome of the CLAVELA Phase III study of elacytarabine in relapsed and refractory acute myeloid leukaemia (AML). The study compares elacytarabine against commonly used but non-standard therapies. Approval requires an odds ratio of 0.7 or better; equivalent to median survival of about 4.5 months vs 3.0 months on other therapies. If this is reached, elacytarabine would be the only approved relapsed/refractory AML therapy. Clavis aims to enter US and Asian deals by the end 2013, with a direct EU launch starting in Germany and Sweden by early 2015. If CLAVELA fails, Clavis will be wound down, with little cash for shareholders although any returns will be maximised.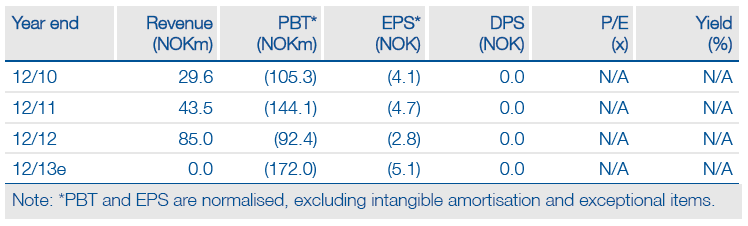
0BInvestment case for third-line elacytarabine
The Phase II data showed an 18% vs 4% remission rate against historic controls. The mechanism is based on improved drug delivery rather than on overcoming drug resistance. Clavis intends to sell direct in the EU with a 50-person team. The EU has about 16,400 AML patients a year of whom 8,000 will be third-line. At €25,000 per year, the EU third-line market at 100% penetration is worth about €200m. This could be very profitable. Clavis will extend into first-line AML and gain US royalties. The US market is about 7,500 third-line patients, and a price of $45k is assumed.
1BFinancials: More funding on success, little back on failure
Cash at 31 December was NOK223m. Net cash after all obligations is NOK130m. If CLAVELA fails, there is enough cash for an orderly liquidation, but shareholders would get a minimal payment. A successful CLAVELA outcome may need a further funding as Clavis would have FDA and EMA regulatory costs to prepare for US and EU launches. Any deal fees would be late in 2013 or into 2014. Clavis will want to maximise royalties rather than take large upfront payments. A third-line label might be extended to first-line AML; Phase II results in combination therapy have been promising.
The core patent expires in April 2014, but orphan protection extends to 2021 in the US and 2024 in the EU. The ability to replace cytarabine depends on data strength and pricing. The current share price assumes success since failure has zero value. If CLAVELA succeeds, Clavis can rebuild its pipeline by using deal fees to acquire underfunded cancer projects from other companies. Assuming regulatory approvals and a US partner at 30% royalty, a rough indicative value is NOK6-7/share. Profits could peak in 2021 at around NOK150-200m with a 30% penetration and a US price of $45,000. The 2013 forecast is approximate, and no 2014 forecast is made since this depends on the CLAVELA outcome.
To Read the Entire Report Please Click on the pdf File Below.
- English (UK)
- English (India)
- English (Canada)
- English (Australia)
- English (South Africa)
- English (Philippines)
- English (Nigeria)
- Deutsch
- Español (España)
- Español (México)
- Français
- Italiano
- Nederlands
- Português (Portugal)
- Polski
- Português (Brasil)
- Русский
- Türkçe
- العربية
- Ελληνικά
- Svenska
- Suomi
- עברית
- 日本語
- 한국어
- 简体中文
- 繁體中文
- Bahasa Indonesia
- Bahasa Melayu
- ไทย
- Tiếng Việt
- हिंदी
Clavis Pharma FY12 Results
Published 02/19/2013, 11:34 PM
Updated 07/09/2023, 06:31 AM
Clavis Pharma FY12 Results
Ragnarök looms
Latest comments
Loading next article…
Install Our App
Risk Disclosure: Trading in financial instruments and/or cryptocurrencies involves high risks including the risk of losing some, or all, of your investment amount, and may not be suitable for all investors. Prices of cryptocurrencies are extremely volatile and may be affected by external factors such as financial, regulatory or political events. Trading on margin increases the financial risks.
Before deciding to trade in financial instrument or cryptocurrencies you should be fully informed of the risks and costs associated with trading the financial markets, carefully consider your investment objectives, level of experience, and risk appetite, and seek professional advice where needed.
Fusion Media would like to remind you that the data contained in this website is not necessarily real-time nor accurate. The data and prices on the website are not necessarily provided by any market or exchange, but may be provided by market makers, and so prices may not be accurate and may differ from the actual price at any given market, meaning prices are indicative and not appropriate for trading purposes. Fusion Media and any provider of the data contained in this website will not accept liability for any loss or damage as a result of your trading, or your reliance on the information contained within this website.
It is prohibited to use, store, reproduce, display, modify, transmit or distribute the data contained in this website without the explicit prior written permission of Fusion Media and/or the data provider. All intellectual property rights are reserved by the providers and/or the exchange providing the data contained in this website.
Fusion Media may be compensated by the advertisers that appear on the website, based on your interaction with the advertisements or advertisers.
Before deciding to trade in financial instrument or cryptocurrencies you should be fully informed of the risks and costs associated with trading the financial markets, carefully consider your investment objectives, level of experience, and risk appetite, and seek professional advice where needed.
Fusion Media would like to remind you that the data contained in this website is not necessarily real-time nor accurate. The data and prices on the website are not necessarily provided by any market or exchange, but may be provided by market makers, and so prices may not be accurate and may differ from the actual price at any given market, meaning prices are indicative and not appropriate for trading purposes. Fusion Media and any provider of the data contained in this website will not accept liability for any loss or damage as a result of your trading, or your reliance on the information contained within this website.
It is prohibited to use, store, reproduce, display, modify, transmit or distribute the data contained in this website without the explicit prior written permission of Fusion Media and/or the data provider. All intellectual property rights are reserved by the providers and/or the exchange providing the data contained in this website.
Fusion Media may be compensated by the advertisers that appear on the website, based on your interaction with the advertisements or advertisers.
© 2007-2024 - Fusion Media Limited. All Rights Reserved.