On 23 August, Cantargia AB (ST:CANTA) announced fresh preclinical in vivo data on CAN04 in bladder cancer, which suggests an opportunity to explore this indication. The company also recently announced that the CAN04 monotherapy arm is now fully enrolled (n=20) and these patients are receiving doses of 10mg/kg. Due to the fast enrolment, Cantargia has decided to enrol an additional monotherapy cohort to test a higher dose of 15mg/kg (n=12). Efficacy and biomarker data from the first 20 patients are expected in Q419. Preparations for its US study are underway – specific timelines were not provided, but we expect more news in this regard this year. Our valuation is virtually unchanged at SEK2.65bn or SEK36.4/share.
Fresh preclinical data: Opportunity in bladder cancer
This is the first preclinical data Cantargia has released on bladder cancer, and the first time the company is discussing it as a potential indication for CAN04 development. Results from immunohistochemistry carried out on tumour samples from 15 patients demonstrated that around 80% of the tumour samples contained IL1RAP positive cells (vs 85% NSCLC, 86% pancreatic cancer, 87% melanoma in a previous study). It was also able to demonstrate single agent activity in an in vivo mouse model with a functioning immune system, where the tumour cells overexpress IL1RAP. Cantargia also recently extended its collaboration with BioWa for its technology POTELLIGENT, which is important for Cantargia’s antibodies since it enhances antibody dependent cellular cytotoxicity (ADCC).
Financials: Q219 results, long cash runway to H121
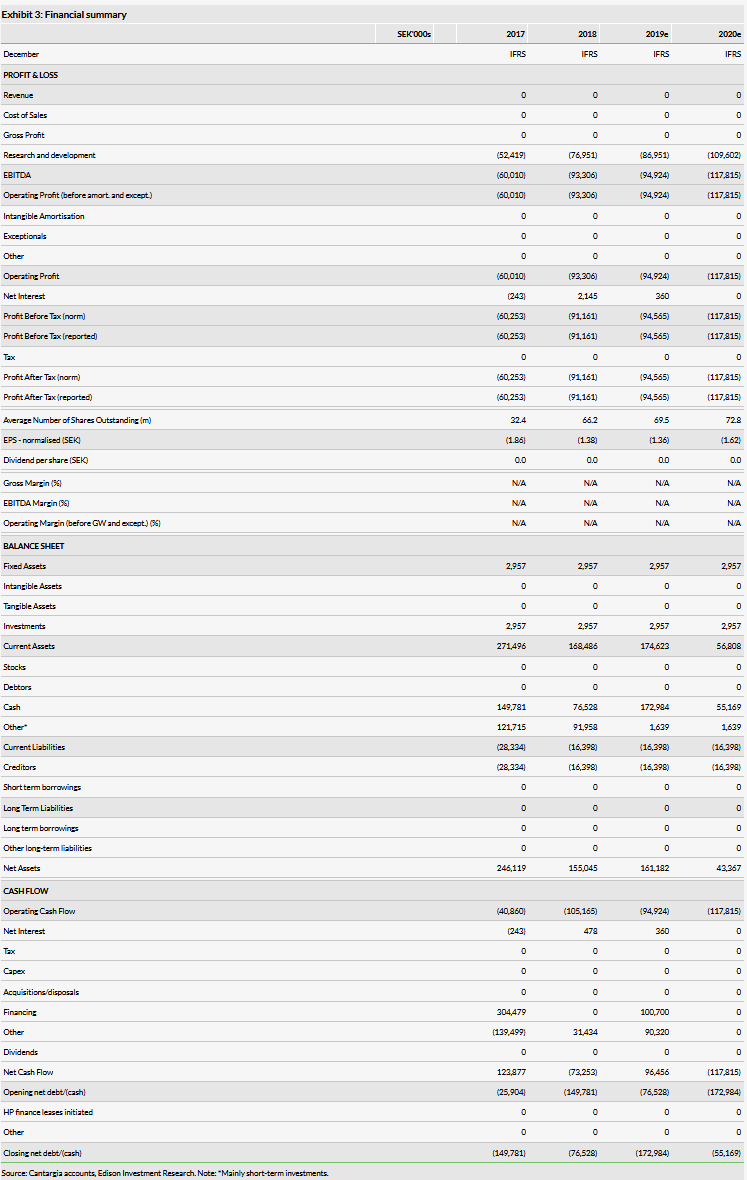
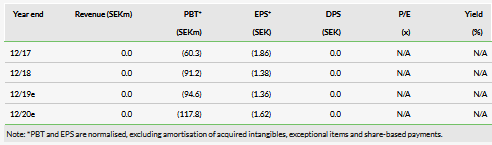
With its Q219 results, Cantargia reported an operating loss of SEK25.2m vs SEK28.6m in Q218. R&D costs in Q219 were SEK20.8m vs SEK22.1m in Q218. In March 2019, Cantargia raised SEK106m (gross) in a directed share issue, primarily by long-term institutional investors. This extended the cash runway to H121 (in line with management guidance). The current net cash position is SEK219.2m (including short-term investments) vs SEK251.2m at end-Q119.
Valuation: SEK2.65bn or SEK36.4/share
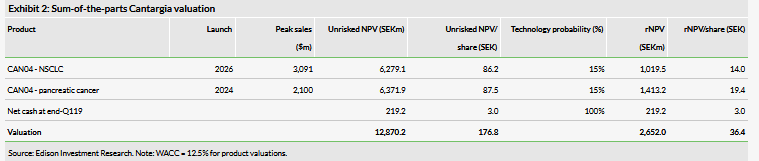
We value Cantargia at SEK2.65bn or SEK36.4/share vs SEK2.62bn or SEK36.0/share previously (Exhibit 2). The slight increase in total NPV is due to rolling our model forward, which was offset by the lower net cash position. We make no changes to the assumptions described in previous reports and in detail in our initiation report. The next key catalyst for the share price will be the Phase IIa CANFOUR data in early 2020.
Business description
Cantargia is a clinical-stage biotechnology company based in Sweden, established in 2009 and listed on the Nasdaq Stockholm main market. It is developing two antibodies against IL1RAP, nidanilimab (CAN04) and CANxx. Nidanilimab is being studied in a Phase I/II clinical trial, CANFOUR, in solid tumours focusing on NSCLC and pancreatic cancer.
Data from monotherapy cohort expected Q419
Cantargia recently announced that the CAN04 monotherapy arm is now fully enrolled (n=20) and these patients are receiving doses of 10mg/kg. According to management, enrolment has been faster than expected. So far, the drug has a good safety profile, as in the Phase I part of the study. Around 12 additional patients are expected to be enrolled due to the fast enrolment into the monotherapy arm which will receive 15mg/kg. 60 further patients in total are due to be enrolled in the combination arms. Efficacy and biomarker data from the first 20 patients are expected in Q419.
Update on IL-1 competitive landscape
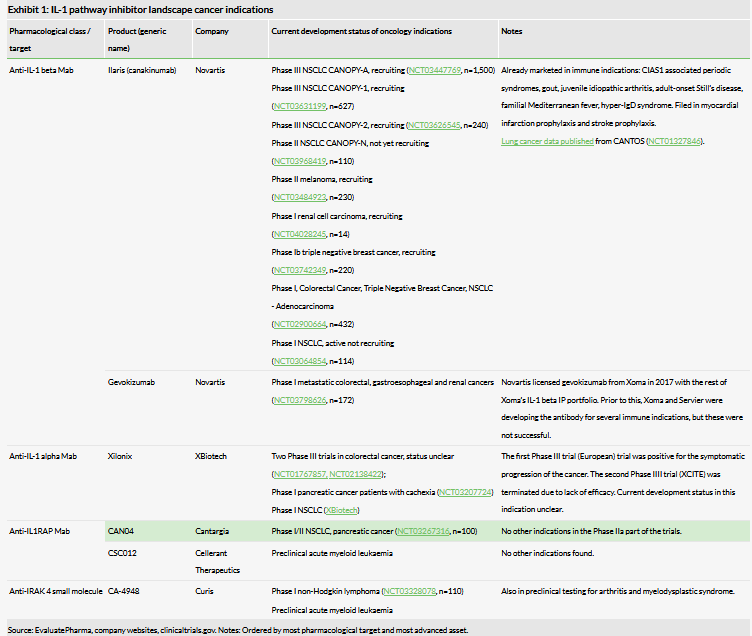
Exhibit 1 shows a summary of all ongoing studies with agents targeting the IL-1 pathway in cancer indications. Novartis now has two IL-1 beta antibodies, canakinumab and gevokizumab, which are being studied in a total of 10 clinical trials. Canakinumab is the most advanced asset and is being studied in three Phase III studies in NSCLC in different patient populations. Completion of these trials is estimated in 2021–22.
More recently, Novartis disclosed new trials in its Q219 results and R&D day presentations, where canakinumab is being studied alongside other agents in triple negative breast cancer, melanoma and RCC. Gevokizumab is being studied in metastatic colorectal, gastroesophageal and renal cancers. In terms of the possible rationale for Novartis choosing these particular cancers, IL-1 is associated with tumour invasiveness and angiogenesis in RCC and breast cancers. XBiotech appears to have restarted Xilonix activity in cancer indications, since it recently announced that it has received funding from the Medical Research Council to conduct a Phase II study in advanced lung, pancreatic and ovarian cancers. We have described previous trials with Xilonix in our initiation report on Cantargia.
For the moment we do not include bladder cancer in our valuation, since it is still pre-clinical but will revise our valuation once we have more visibility on clinical development timelines.