AstraZeneca’s discontinuation of AZD9773/CytoFab after the failure of a Phase IIb study in severe sepsis/septic shock has a material impact on BTG’s valuation, but given the larger recent share price movement, the investment case remains strong. Removing CytoFab’s contribution and making certain other changes reduces our valuation from 430p to 398p per share. This suggests there is an almost 15% upside to a valuation that is largely underpinned by the DCF value of a solid revenue-generating business while, by biotech standards, BTG has a low risk profile.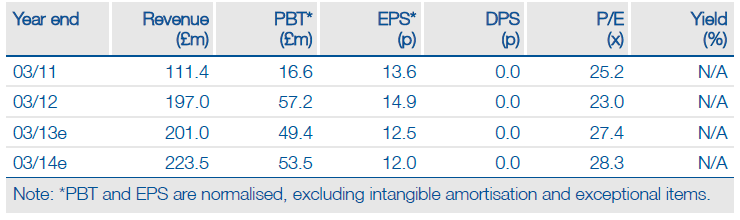
0BCytoFab fails in the challenging sepsis indication
AstraZeneca has discontinued AZD9773/CytoFab after the failure of its 300-patient Phase IIb study in severe sepsis/septic shock. Although disappointing, the outcome of the study is a reflection of the challenging nature of the sepsis indication. CytoFab was carried at a low probability in our model, although the attractive economics of the licensing deal meant it still made a material contribution (£136m) to the valuation.
1BBenefix windfall, Zytiga sales tracking $1.2bn/year
BTG recently disclosed a further windfall royalty on Benefix, which added c £10m to its FY13 revenue guidance to £190-200m; Edison’s model suggests revenues may come in slightly higher at £201m. Furthermore, reported sales of Zytiga by Johnson & Johnson suggest this product is on track to achieve $1.2bn sales this year. It thus has the potential to make a significant (and probably under-appreciated) contribution to BTG’s FY13 revenues.
2BCore business performing well
BTG’s core direct sales operations (CroFab, DigiFab and Bead products) continue to perform well. The next key milestone is likely to be the planned filing of Varisolve, due in Q412.
3BValuation: Fair value now 398p per share
Removing the CytoFab contribution and updating the valuation to reflect the Benefix windfall and using FY13 year-end cash (£144m) suggests a valuation of £1.3bn or 398p per share. This compares with the previously published £1.4bn or 440p/share. Thus we consider BTG offers an attractive investment proposition, with the current share price offering 14% upside to a valuation that is supported by the DCF value of its core business activities and a low risk profile by biotech standards.
To Read the Entire Report Please Click on the pdf File Below.
- English (UK)
- English (India)
- English (Canada)
- English (Australia)
- English (South Africa)
- English (Philippines)
- English (Nigeria)
- Deutsch
- Español (España)
- Español (México)
- Français
- Italiano
- Nederlands
- Português (Portugal)
- Polski
- Português (Brasil)
- Русский
- Türkçe
- العربية
- Ελληνικά
- Svenska
- Suomi
- עברית
- 日本語
- 한국어
- 简体中文
- 繁體中文
- Bahasa Indonesia
- Bahasa Melayu
- ไทย
- Tiếng Việt
- हिंदी
BTG Cyto Fab Study Failure
Published 08/09/2012, 06:50 AM
Updated 07/09/2023, 06:31 AM
BTG Cyto Fab Study Failure
Look beyond the short-term shock
Latest comments
Loading next article…
Install Our App
Risk Disclosure: Trading in financial instruments and/or cryptocurrencies involves high risks including the risk of losing some, or all, of your investment amount, and may not be suitable for all investors. Prices of cryptocurrencies are extremely volatile and may be affected by external factors such as financial, regulatory or political events. Trading on margin increases the financial risks.
Before deciding to trade in financial instrument or cryptocurrencies you should be fully informed of the risks and costs associated with trading the financial markets, carefully consider your investment objectives, level of experience, and risk appetite, and seek professional advice where needed.
Fusion Media would like to remind you that the data contained in this website is not necessarily real-time nor accurate. The data and prices on the website are not necessarily provided by any market or exchange, but may be provided by market makers, and so prices may not be accurate and may differ from the actual price at any given market, meaning prices are indicative and not appropriate for trading purposes. Fusion Media and any provider of the data contained in this website will not accept liability for any loss or damage as a result of your trading, or your reliance on the information contained within this website.
It is prohibited to use, store, reproduce, display, modify, transmit or distribute the data contained in this website without the explicit prior written permission of Fusion Media and/or the data provider. All intellectual property rights are reserved by the providers and/or the exchange providing the data contained in this website.
Fusion Media may be compensated by the advertisers that appear on the website, based on your interaction with the advertisements or advertisers.
Before deciding to trade in financial instrument or cryptocurrencies you should be fully informed of the risks and costs associated with trading the financial markets, carefully consider your investment objectives, level of experience, and risk appetite, and seek professional advice where needed.
Fusion Media would like to remind you that the data contained in this website is not necessarily real-time nor accurate. The data and prices on the website are not necessarily provided by any market or exchange, but may be provided by market makers, and so prices may not be accurate and may differ from the actual price at any given market, meaning prices are indicative and not appropriate for trading purposes. Fusion Media and any provider of the data contained in this website will not accept liability for any loss or damage as a result of your trading, or your reliance on the information contained within this website.
It is prohibited to use, store, reproduce, display, modify, transmit or distribute the data contained in this website without the explicit prior written permission of Fusion Media and/or the data provider. All intellectual property rights are reserved by the providers and/or the exchange providing the data contained in this website.
Fusion Media may be compensated by the advertisers that appear on the website, based on your interaction with the advertisements or advertisers.
© 2007-2024 - Fusion Media Limited. All Rights Reserved.