A recommendation for approval in Europe of alcohol dependence drug Selincro and positive Phase IIb data for Parkinson’s disease candidate tozadenant are breakthrough events that enhance Biotie’s (F8S.BE) investment case. The next key catalyst is UCB’s decision in Q113 on whether to advance tozadenant into Phase III trials, potentially triggering a c €25m milestone. A positive verdict by UCB could therefore enable Biotie to advance its pipeline candidates, such as SYN120 for cognitive disorders, to greater valuation inflection points. With Selincro to be launched in Europe by partner Lundbeck in mid-2013, Biotie is entering a transformational period.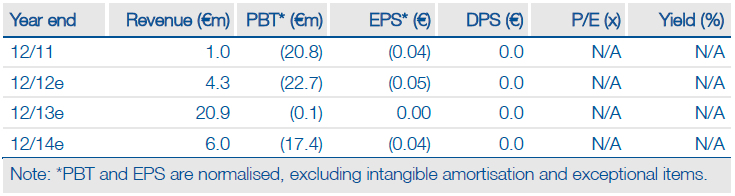
Selincro: EU CHMP positive opinion
On 13 December 2012 Europe’s recommendation committee adopted a positive opinion on Selincro (nalmefene) 18.6mg tablets to be taken as-needed to reduce alcohol consumption (there are an estimated 16 million alcoholics in Europe). Selincro reduced consumption by >60% on average in pivotal studies and offers a new and more realistic treatment option for many alcohol dependents compared to existing drug therapies, which are minimally effective at maintaining abstinence from drinking.
Selincro: Lundbeck to launch mid-2013
Assuming full EC approval in Q113, Lundbeck will start to roll-out Selincro in key European markets in mid-2013, triggering milestones (~€10m over 18 months) and royalties on sales (~15%). Given Selincro’s new treatment concept, initial uptake is likely to be modest but should accelerate significantly, and we now estimate peak sales of €320m by the end of Selincro’s 10 years of market exclusivity in Europe.
Tozadenant: Positive headline data, awaiting UCB decision
Also in December, Biotie announced positive headline Phase IIb (n=420) data for tozadenant in significantly reducing ‘off-time’ in Parkinson’s disease patients suffering wearing off fluctuations on levodopa. Multiple secondary, non-motor endpoints were also met. Full efficacy and safety data were not revealed, but we are hopeful that UCB will decide to take tozadenant into Phase III, triggering a significant milestone (~€25m).
Valuation: €242m with near-term upside potential
Following fresh guidance from Lundbeck on potential pricing and usage rates, we now include detailed forecasts for Selincro which produces a peak sales estimate of €320m. Our revised model for Biotie now yields a total valuation of €242m, which also includes €35m estimated end-2012 cash and an increase in tozadenant’s probability – UCB’s support would increase our valuation to c €255m.
To Read the Entire Report Please Click on the pdf File Below.
- English (UK)
- English (India)
- English (Canada)
- English (Australia)
- English (South Africa)
- English (Philippines)
- English (Nigeria)
- Deutsch
- Español (España)
- Español (México)
- Français
- Italiano
- Nederlands
- Português (Portugal)
- Polski
- Português (Brasil)
- Русский
- Türkçe
- العربية
- Ελληνικά
- Svenska
- Suomi
- עברית
- 日本語
- 한국어
- 简体中文
- 繁體中文
- Bahasa Indonesia
- Bahasa Melayu
- ไทย
- Tiếng Việt
- हिंदी
Biotie Therapies: 2 Breakthrough Events Enhance Investment Case
Published 01/07/2013, 01:45 AM
Updated 07/09/2023, 06:31 AM
Biotie Therapies: 2 Breakthrough Events Enhance Investment Case
A perfect tonic for 2013
3rd party Ad. Not an offer or recommendation by Investing.com. See disclosure here or
remove ads
.
Latest comments
Install Our App
Risk Disclosure: Trading in financial instruments and/or cryptocurrencies involves high risks including the risk of losing some, or all, of your investment amount, and may not be suitable for all investors. Prices of cryptocurrencies are extremely volatile and may be affected by external factors such as financial, regulatory or political events. Trading on margin increases the financial risks.
Before deciding to trade in financial instrument or cryptocurrencies you should be fully informed of the risks and costs associated with trading the financial markets, carefully consider your investment objectives, level of experience, and risk appetite, and seek professional advice where needed.
Fusion Media would like to remind you that the data contained in this website is not necessarily real-time nor accurate. The data and prices on the website are not necessarily provided by any market or exchange, but may be provided by market makers, and so prices may not be accurate and may differ from the actual price at any given market, meaning prices are indicative and not appropriate for trading purposes. Fusion Media and any provider of the data contained in this website will not accept liability for any loss or damage as a result of your trading, or your reliance on the information contained within this website.
It is prohibited to use, store, reproduce, display, modify, transmit or distribute the data contained in this website without the explicit prior written permission of Fusion Media and/or the data provider. All intellectual property rights are reserved by the providers and/or the exchange providing the data contained in this website.
Fusion Media may be compensated by the advertisers that appear on the website, based on your interaction with the advertisements or advertisers.
Before deciding to trade in financial instrument or cryptocurrencies you should be fully informed of the risks and costs associated with trading the financial markets, carefully consider your investment objectives, level of experience, and risk appetite, and seek professional advice where needed.
Fusion Media would like to remind you that the data contained in this website is not necessarily real-time nor accurate. The data and prices on the website are not necessarily provided by any market or exchange, but may be provided by market makers, and so prices may not be accurate and may differ from the actual price at any given market, meaning prices are indicative and not appropriate for trading purposes. Fusion Media and any provider of the data contained in this website will not accept liability for any loss or damage as a result of your trading, or your reliance on the information contained within this website.
It is prohibited to use, store, reproduce, display, modify, transmit or distribute the data contained in this website without the explicit prior written permission of Fusion Media and/or the data provider. All intellectual property rights are reserved by the providers and/or the exchange providing the data contained in this website.
Fusion Media may be compensated by the advertisers that appear on the website, based on your interaction with the advertisements or advertisers.
© 2007-2024 - Fusion Media Limited. All Rights Reserved.