Biotie Therapies' (F8S.BE) retention of SYN120 rights after Roche (ROG.VX) decided not to opt-in to a development license means the company now has three unencumbered Phase IIready assets available for global licensing. A deal on any of these could extend Biotie’s current cash runway beyond early 2013, although the company may explore other options as additional funds will likely need to be raised this year. The next key catalyst with known timing is the European approval decision for alcohol dependence therapy Selincro, expected by year-end. Approval could mean a Q113 launch, which would trigger an undisclosed milestone payment from partner Lundbeck.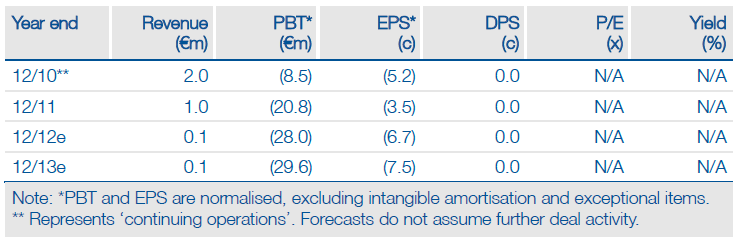
SYN120: Rights returned for strategic portfolio reasons
Various large pharma/CNS specialists are investigating 5-HT6 antagonists, which have potential in cognitive impairment; Lundbeck has recently achieved proof of concept in Alzheimer’s disease. SYN120 is safe/well tolerated, has a wide therapeutic window and confirmed dosing for Phase II. Biotie has been approached by interested parties, although deal timing is uncertain. Other Phase II-ready assets available for partnering include BTT-1023 (H2 start of clinical studies in fibrotic disorders) and ronomilast.
Licensed programmes: Selincro and tozadenant
Commercial success of the two partnered projects has potential to transform Biotie. The EMA approval decision for Selincro (alcohol dependence) by end-2012 should catalyse the share price; potential Q113 launch would trigger a Lundbeck milestone. The next news flow for UCB-partnered tozadenant is the read-out of the Phase IIb trial in Parkinson’s disease (which recently completed enrolment) around end-2012.
Financials: Cash into 2013 with potential for deals
Q1 cash of €25m represents funds into 2013 in the absence of additional receipts. Upfront payments on new licensing deals, milestones from existing partners (Lundbeck on Selincro EU launch, UCB on tozadenant Phase IIb data/Phase III start), and/or an equity raise in 2012 could address Biotie’s funding requirement.
Valuation: €243m rNPV, current EV of €115m
Our new rNPV of €243m (previously €250m) reflects BTT-1023’s re-positioning as a potential fibrotic disease therapy. This valuation only includes the lead indications of Biotie’s five core assets and compares with a €115m EV (excluding the Tekes financial liability, which is only repayable on profitability). Significant potential milestones from Lundbeck and UCB are not captured in this valuation, and thus represent upside.
To Read the Entire Report Please Click on the pdf File Below.
- English (UK)
- English (India)
- English (Canada)
- English (Australia)
- English (South Africa)
- English (Philippines)
- English (Nigeria)
- Deutsch
- Español (España)
- Español (México)
- Français
- Italiano
- Nederlands
- Português (Portugal)
- Polski
- Português (Brasil)
- Русский
- Türkçe
- العربية
- Ελληνικά
- Svenska
- Suomi
- עברית
- 日本語
- 한국어
- 简体中文
- 繁體中文
- Bahasa Indonesia
- Bahasa Melayu
- ไทย
- Tiếng Việt
- हिंदी
Biotie Therapies Update: SYN120 Rights Retained
Published 07/08/2012, 02:25 AM
Updated 07/09/2023, 06:31 AM
Biotie Therapies Update: SYN120 Rights Retained
Assets available for partnering
3rd party Ad. Not an offer or recommendation by Investing.com. See disclosure here or
remove ads
.
Latest comments
Install Our App
Risk Disclosure: Trading in financial instruments and/or cryptocurrencies involves high risks including the risk of losing some, or all, of your investment amount, and may not be suitable for all investors. Prices of cryptocurrencies are extremely volatile and may be affected by external factors such as financial, regulatory or political events. Trading on margin increases the financial risks.
Before deciding to trade in financial instrument or cryptocurrencies you should be fully informed of the risks and costs associated with trading the financial markets, carefully consider your investment objectives, level of experience, and risk appetite, and seek professional advice where needed.
Fusion Media would like to remind you that the data contained in this website is not necessarily real-time nor accurate. The data and prices on the website are not necessarily provided by any market or exchange, but may be provided by market makers, and so prices may not be accurate and may differ from the actual price at any given market, meaning prices are indicative and not appropriate for trading purposes. Fusion Media and any provider of the data contained in this website will not accept liability for any loss or damage as a result of your trading, or your reliance on the information contained within this website.
It is prohibited to use, store, reproduce, display, modify, transmit or distribute the data contained in this website without the explicit prior written permission of Fusion Media and/or the data provider. All intellectual property rights are reserved by the providers and/or the exchange providing the data contained in this website.
Fusion Media may be compensated by the advertisers that appear on the website, based on your interaction with the advertisements or advertisers.
Before deciding to trade in financial instrument or cryptocurrencies you should be fully informed of the risks and costs associated with trading the financial markets, carefully consider your investment objectives, level of experience, and risk appetite, and seek professional advice where needed.
Fusion Media would like to remind you that the data contained in this website is not necessarily real-time nor accurate. The data and prices on the website are not necessarily provided by any market or exchange, but may be provided by market makers, and so prices may not be accurate and may differ from the actual price at any given market, meaning prices are indicative and not appropriate for trading purposes. Fusion Media and any provider of the data contained in this website will not accept liability for any loss or damage as a result of your trading, or your reliance on the information contained within this website.
It is prohibited to use, store, reproduce, display, modify, transmit or distribute the data contained in this website without the explicit prior written permission of Fusion Media and/or the data provider. All intellectual property rights are reserved by the providers and/or the exchange providing the data contained in this website.
Fusion Media may be compensated by the advertisers that appear on the website, based on your interaction with the advertisements or advertisers.
© 2007-2024 - Fusion Media Limited. All Rights Reserved.