UCB’s decision to support further development of Biotie’s (OMX: BTH1V) Phase IIb Parkinson’s disease candidate tozadenant provides encouraging endorsement and a $20m upfront fee. Biotie will now conduct the Phase III studies – to start in H115 – but UCB will effectively fund the trials through additional payments (low triple-digit $m) over the next six years. Tozadenant is now Biotie’s primary focus, and with cash of €49m coupled with milestones/royalties to flow in from Lundbeck’s EU sales of alcohol-dependence drug Selincro, the company is well positioned to advance tozadenant and to conduct a portfolio review that may bring in new opportunities.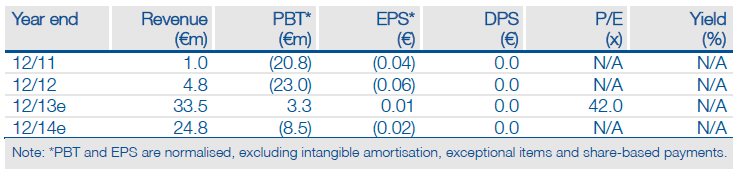
Role reversal
Prior to the revised deal, our assumption was that UCB would take over full responsibility for further clinical and regulatory development of tozadenant. The flipping of responsibility onto Biotie is a reflection of UCB’s strategy to outsource/de-risk its pipeline, and is also an endorsement of Biotie’s competence in conducting the Phase IIb study. Biotie reported positive Phase IIb headline data, across multiple endpoints, in December and detailed results will be presented in a late-breaking abstract at the American Academy of Neurology (AAN) meeting (16-23 March).
Costs covered
In return for taking on the cost and responsibility for running the tozadenant pivotal trials, UCB has agreed to make additional payments (low triple-digit $m) over the next six years, effectively covering Biotie’s R&D costs on tozadenant. These payments are additional to the $340m potential milestones (clinical, regulatory and commercial) Biotie could also receive under the terms of the original deal with UCB in 2010.
Portfolio review
Biotie will review its portfolio, and potential new strategic opportunities, through Q213 and has already decided to write-off ronomilast (a PDE4 inhibitor for COPD) after failing to secure a partner (€3.4m non-cash impairment charge in Q412). Biotie is still seeking partners for SYN120 (psychosis disorders) and BTT-1023 (fibrotic diseases).
Valuation: Revised to €215m after pipeline review
Following the write-down of ronomilast and Biotie’s ongoing portfolio review, we have also prudently removed BTT-1023 from our valuation model. Our revised valuation, based purely on Selincro, tozadenant, SYN120 and €49m estimated cash (€34m at end-2012 + €15m UCB upfront), is now €215m (previously €242m). Detailed Phase IIb tozadenant data provides near-term upside potential (to €228m on raising tozadenant’s probability of success to 65%).
To Read the Entire Report Please Click on the pdf File Below.
- English (UK)
- English (India)
- English (Canada)
- English (Australia)
- English (South Africa)
- English (Philippines)
- English (Nigeria)
- Deutsch
- Español (España)
- Español (México)
- Français
- Italiano
- Nederlands
- Português (Portugal)
- Polski
- Português (Brasil)
- Русский
- Türkçe
- العربية
- Ελληνικά
- Svenska
- Suomi
- עברית
- 日本語
- 한국어
- 简体中文
- 繁體中文
- Bahasa Indonesia
- Bahasa Melayu
- ไทย
- Tiếng Việt
- हिंदी
Biotie's Tozadenant Enters Pivotal territory With UCB Deal
Published 03/06/2013, 07:18 AM
Updated 07/09/2023, 06:31 AM
Biotie's Tozadenant Enters Pivotal territory With UCB Deal
Tozadenant enters pivotal territory
3rd party Ad. Not an offer or recommendation by Investing.com. See disclosure here or
remove ads
.
Latest comments
Install Our App
Risk Disclosure: Trading in financial instruments and/or cryptocurrencies involves high risks including the risk of losing some, or all, of your investment amount, and may not be suitable for all investors. Prices of cryptocurrencies are extremely volatile and may be affected by external factors such as financial, regulatory or political events. Trading on margin increases the financial risks.
Before deciding to trade in financial instrument or cryptocurrencies you should be fully informed of the risks and costs associated with trading the financial markets, carefully consider your investment objectives, level of experience, and risk appetite, and seek professional advice where needed.
Fusion Media would like to remind you that the data contained in this website is not necessarily real-time nor accurate. The data and prices on the website are not necessarily provided by any market or exchange, but may be provided by market makers, and so prices may not be accurate and may differ from the actual price at any given market, meaning prices are indicative and not appropriate for trading purposes. Fusion Media and any provider of the data contained in this website will not accept liability for any loss or damage as a result of your trading, or your reliance on the information contained within this website.
It is prohibited to use, store, reproduce, display, modify, transmit or distribute the data contained in this website without the explicit prior written permission of Fusion Media and/or the data provider. All intellectual property rights are reserved by the providers and/or the exchange providing the data contained in this website.
Fusion Media may be compensated by the advertisers that appear on the website, based on your interaction with the advertisements or advertisers.
Before deciding to trade in financial instrument or cryptocurrencies you should be fully informed of the risks and costs associated with trading the financial markets, carefully consider your investment objectives, level of experience, and risk appetite, and seek professional advice where needed.
Fusion Media would like to remind you that the data contained in this website is not necessarily real-time nor accurate. The data and prices on the website are not necessarily provided by any market or exchange, but may be provided by market makers, and so prices may not be accurate and may differ from the actual price at any given market, meaning prices are indicative and not appropriate for trading purposes. Fusion Media and any provider of the data contained in this website will not accept liability for any loss or damage as a result of your trading, or your reliance on the information contained within this website.
It is prohibited to use, store, reproduce, display, modify, transmit or distribute the data contained in this website without the explicit prior written permission of Fusion Media and/or the data provider. All intellectual property rights are reserved by the providers and/or the exchange providing the data contained in this website.
Fusion Media may be compensated by the advertisers that appear on the website, based on your interaction with the advertisements or advertisers.
© 2007-2024 - Fusion Media Limited. All Rights Reserved.