BioPharma Credit PLC (LON:BPCR) recently announced that it has entered into an agreement to provide a US$80m senior secured loan to BioDelivery Sciences International (BDSI), a Nasdaq-listed commercial-stage pharma company. BDSI’s main assets include Belbuca, an FDA-approved partial opioid agonist classified as a Schedule III drug, as well as Symproic, an FDA-approved drug for the treatment of opioid-induced constipation (OIC). This agreement represents BPCR’s first investment this year. Moreover, it invested US$25m in BDSI’s recent share issue, which constitutes BPCR’s first equity investment since inception. We estimate that BPCR still has around US$500m in uncommitted cash available for further deals.

Loan terms broadly in line with previous transactions
BPCR has agreed to provide US$80m of debt funding through a senior secured loan (secured on substantially all of BDSI’s assets). US$60m was provided on 28 May, with the additional US$20m tranche available until May 2020. The loan has a coupon rate of Libor plus 7.5% and BPCR will also receive a funding fee of 2.0%. These terms are in line with BPCR’s last deal (Amicus) which concluded in September 2018 and is broadly comparable with the earlier Tesaro transaction. The loan is interest-only for the first three years.
BPCR’s first equity investment
BPCR also acquired a US$25m equity stake during BDSI’s secondary public offering in April 2019 (representing 50% of the new share issue) priced at US$5.00/share (vs the last close which was US$4.24). BPCR’s equity stake now represents c 6% of BDSI’s share capital. As per its investment policy, its total equity exposure can be up to 15% of BPCR’s gross assets. Overall, the BDSI deal is a somewhat smaller transaction for BPCR, as the combined debt and equity investment (including the second US$20m tranche not yet funded) equals c 7% of BPCR’s NAV as at end-April 2019. This compares with the share of each of the other senior secured loans in the company’s portfolio, at c 9–12%. However, we appreciate that it moderately reduces BPCR’s current high cash position.
Valuation: Offers a c 7% dividend yield
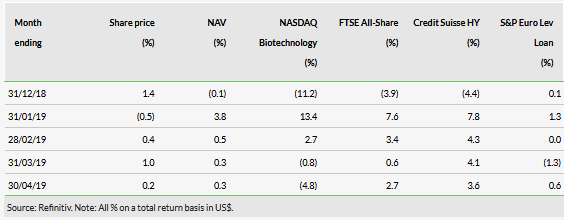
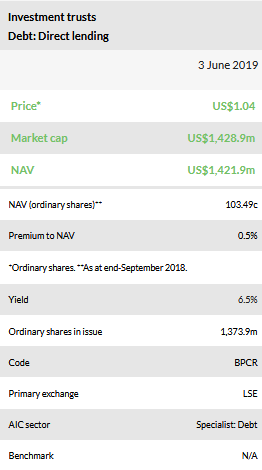
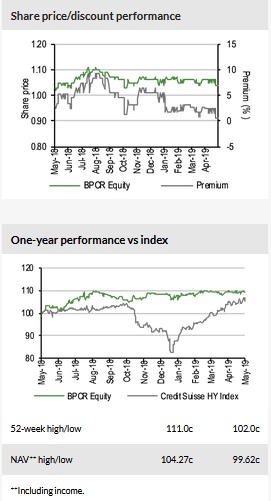
At 30 May 2019, BPCR’s shares are trading at a minor 0.5% premium to its last reported NAV (as at end-April 2019). Including the DPS of 1.8c payable in June, the shares offer a c 7% trailing dividend yield, in line with BPCR’s target
Transaction rationale
BDSI: Product ramp up supports funding measures
BPCR’s transaction with BDSI follows the solid ramp-up in sales of Belbuca (up 134% y-o-y to US$18.7m in Q119) and BDSI’s acquisition of the commercialisation rights to Symproic in April this year. Belbuca (which was developed by BDSI in partnership with Endo) received FDA approval in October 2015. BDSI subsequently re-acquired full commercial rights to the drug in January 2017 when Endo discontinued its commercial efforts with respect to its branded pain business.
BDSI raised its FY19 sales guidance for Belbuca following the Q119 results announcement to US$83–88m (implying y-o-y growth at 80–91%) from US$80–85m previously. The Evaluate Pharma consensus for FY19 currently stands at US$80m, with further growth expected for FY20 and FY21 of 39% y-o-y (to US$111m) and 21% (to US$134m), respectively. The company also confirmed group sales guidance for FY19 of US$92–100m (including US$7–9m attributable to Symproic). The ramp up in Belbuca sales has allowed BDSI to refinance the more expensive US$61.8m loan from CRG Services (fixed coupon rate of 12.5%) with the BPCR loan and raise US$50m gross from an equity offering in April.
Demand for Schedule III pain relief drugs is likely to remain high
The opioid epidemic in the US and other markets is significant and results in an estimated 130 deaths from overdose every day according to the US National Institutes of Health. The cause of this opioid crisis is partly due to the rise in surgical procedures and the number of cancer patients. While efforts to reduce opioid prescriptions have had an effect, some sources suggest that this is due to a reclassification of some drugs. Nevertheless, demand for so-called Schedule III opioid formulations like Belbuca that, by virtue of their drug delivery systems are less able to be abused or diverted, but still treat chronic pain effectively, is likely to remain high in patients requiring pain management. In the same way, many cancer and surgical patients suffer the side effects of opioid pain medication as these drugs bind to the opioid receptors in the gut leading to OIC, which delays a patient’s discharge from hospital. Drugs such as Symproic have been developed to address this indication and their use can be associated with a positive return on investment at the hospital level, as the earlier discharge of patients frees up beds for more admissions.
Significant dry powder still available for further deals
At end-April 2019, BPCR had US$724.3m in cash (or 51% of its NAV). In part this is due to the acquisition of Tesaro (BPCR’s largest borrower at end-2018) by GlaxoSmithKline in early 2019, which triggered the loan repayment based on a change of control clause. Together with substantial make-whole/prepayment fees, this translated into a cash inflow of US$369.9m to BPCR on its US$322m investment. As a result, the company generated a solid IRR of 28.8% on this investment. The healthy make-whole payment on the Tesaro loan allows for a long reinvestment runway of the returned cash. Moreover, in autumn 2018, the company raised gross proceeds of US$305m in a new share issue. The US$65m invested in BDSI (excluding the second tranche of US$20m) represents a relatively minor part of BPCR’s cash balance, leaving the company with ample dry powder for prospective deals.