Bionomics’ (BNO.AX) acquisition of San Diego-based Eclipse Therapeutics last year brought in new technology and expertise in antibody and cancer stem cells, complementing its existing capabilities in the development of small molecule therapeutics. The company’s lead compound remains BNC105, which is in Phase II trials for renal cell carcinoma and ovarian cancer and has recently become the lead vascular disrupting agent in commercial development. Eclipse added BNC101, a late preclinical antibody against LGR5, a cancer stem cell target. Meanwhile, anti-anxiety compound IW-2143, licensed to Ironwood Pharmaceuticals, is now in a Phase I trial in the US.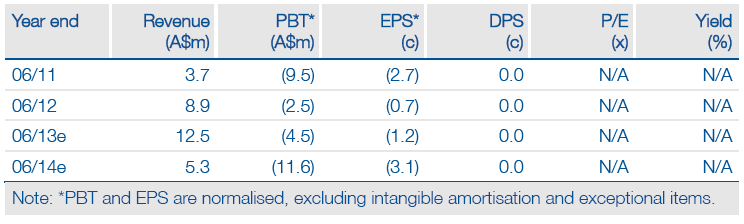
Eclipse adds CSC antibody to oncology pipeline
The Eclipse acquisition gave Bionomics CSC cancer stem cell technology and provides a late preclinical programme, BNC101, which is expected to enter Phase I trials in 2014. BNC101 is a monoclonal antibody to LGR5/GPR49, a target on cancer stem cells particularly associated with colon and gastric cancer.
BNC105 still the main focus, now lead VDA
Meanwhile, the discontinuation of Sanofi’s ombrabulin after an underwhelming Phase III study result, promotes BNC105 to lead position in the vascular disrupting agent class. Bionomics is focused on securing a partner for this drug, which is in a Phase II study in RCC that is now well into its second stage, with near-term completion of enrolment anticipated in H213. Results of the study should be rendered towards the end of CY13. Bionomics plans to initiate a Phase II study of BNC105 in second-line ovarian cancer in combination with carboplatin/gemcitabine in early Q213 following completion of the current Phase I trial in this setting.
Cash to beyond end-2013
Bionomics’ cash at 30 June 2012 was A$17.3m, which should provide funding to around mid-CY14, not including A$4.2m cash from R&D tax incentives. Additionally, near-term milestones for IW-2143 are anticipated.
Valuation: A$275m risk-adjusted NPV
Our risk-adjusted net present value model for Bionomics’ clinical stage R&D programmes, excluding cash, yields a figure of A$275m. We compare this with the current enterprise value (EV) of A$137m (market cap of A$151m less estimated A$14m net cash), highlighting an attractive investment case. It should also be noted that risk-adjusted NPVs rise rapidly as products advance through development and justify higher probabilities of success.
To Read the Entire Report Please Click on the pdf File Below.
- English (UK)
- English (India)
- English (Canada)
- English (Australia)
- English (South Africa)
- English (Philippines)
- English (Nigeria)
- Deutsch
- Español (España)
- Español (México)
- Français
- Italiano
- Nederlands
- Português (Portugal)
- Polski
- Português (Brasil)
- Русский
- Türkçe
- العربية
- Ελληνικά
- Svenska
- Suomi
- עברית
- 日本語
- 한국어
- 简体中文
- 繁體中文
- Bahasa Indonesia
- Bahasa Melayu
- ไทย
- Tiếng Việt
- हिंदी
Bionomics: Acquisition Boosts Oncology Pipeline
Published 02/06/2013, 11:55 PM
Updated 07/09/2023, 06:31 AM
Bionomics: Acquisition Boosts Oncology Pipeline
Eclipse boosts oncology pipeline
3rd party Ad. Not an offer or recommendation by Investing.com. See disclosure here or
remove ads
.
Latest comments
Install Our App
Risk Disclosure: Trading in financial instruments and/or cryptocurrencies involves high risks including the risk of losing some, or all, of your investment amount, and may not be suitable for all investors. Prices of cryptocurrencies are extremely volatile and may be affected by external factors such as financial, regulatory or political events. Trading on margin increases the financial risks.
Before deciding to trade in financial instrument or cryptocurrencies you should be fully informed of the risks and costs associated with trading the financial markets, carefully consider your investment objectives, level of experience, and risk appetite, and seek professional advice where needed.
Fusion Media would like to remind you that the data contained in this website is not necessarily real-time nor accurate. The data and prices on the website are not necessarily provided by any market or exchange, but may be provided by market makers, and so prices may not be accurate and may differ from the actual price at any given market, meaning prices are indicative and not appropriate for trading purposes. Fusion Media and any provider of the data contained in this website will not accept liability for any loss or damage as a result of your trading, or your reliance on the information contained within this website.
It is prohibited to use, store, reproduce, display, modify, transmit or distribute the data contained in this website without the explicit prior written permission of Fusion Media and/or the data provider. All intellectual property rights are reserved by the providers and/or the exchange providing the data contained in this website.
Fusion Media may be compensated by the advertisers that appear on the website, based on your interaction with the advertisements or advertisers.
Before deciding to trade in financial instrument or cryptocurrencies you should be fully informed of the risks and costs associated with trading the financial markets, carefully consider your investment objectives, level of experience, and risk appetite, and seek professional advice where needed.
Fusion Media would like to remind you that the data contained in this website is not necessarily real-time nor accurate. The data and prices on the website are not necessarily provided by any market or exchange, but may be provided by market makers, and so prices may not be accurate and may differ from the actual price at any given market, meaning prices are indicative and not appropriate for trading purposes. Fusion Media and any provider of the data contained in this website will not accept liability for any loss or damage as a result of your trading, or your reliance on the information contained within this website.
It is prohibited to use, store, reproduce, display, modify, transmit or distribute the data contained in this website without the explicit prior written permission of Fusion Media and/or the data provider. All intellectual property rights are reserved by the providers and/or the exchange providing the data contained in this website.
Fusion Media may be compensated by the advertisers that appear on the website, based on your interaction with the advertisements or advertisers.
© 2007-2024 - Fusion Media Limited. All Rights Reserved.