Value of c-Met inhibitor in NSCLC questioned
Detailed data presented at ASCO 2014 on c-Met inhibitors, including Roche’s onartuzumab and ArQule Inc's (NASDAQ:ARQL) tivantinib, failed to show much clinical benefit when a c-Met inhibitor is added to other therapies in NSCLC, and posed the question of whether inhibiting c-Met in this disease setting is clinically meaningful. This, however, should not have reduced tivantinib’s value in HCC, in which two Phase III trials, METIV-HCC and JET-HCC, are progressing in second-line HCC. ArQule ended Q114 with cash and marketable securities of $85.8m, enough to support its operation beyond 2015.
Met inhibition in NSCLC failed to show efficacy
Detailed data of the Phase III METLung trial (stopped prematurely in March 2014 after it failed a pre-planned interim futility analysis) showed that the combination of onartuzumab (an anti-Met mAb) and Tarceva (Roche) did not have any clinical benefit (OS, PFS and ORR) over placebo and Tarceva in NSCLC patients with high c-Met expression status. This was in contrast to OS and PFS benefits seen in the same patient population in a previous Phase II trial. Similarly, the combination of tivantinib and Tarceva (t/T) failed to show any clinical benefit over placebo and Tarceva in previously treated, squamous NSCLC with EGFR wild-type, c-Met high patients. The t/T combination did cause higher incidences of interstitial lung disease (ILD), which caused the ATTENTION trial to be stopped prematurely in August 2012. The lack of clinical benefit of a c-Met inhibitor when added to an EGFR inhibitor (Tarceva is an EGFR inhibitor) in patients with high c-Met expression (patients were specifically selected based on their high c-Met status in both trials) has casted doubts on c-Met as a valid drug target in NSCLC.
No crossover read to tivantinib in HCC
We continue to caution a crossover read of the NSCLC results into that of HCC, and, more specifically, the probability of success of tivantinib in the ongoing Phase III METIV-HCC. The trial was based on a positive Phase II trial in which a statistically significant OS benefit was demonstrated, and tivantinib was compared to a placebo, a lower hurdle than Tarceva, in the two aforementioned trials.
Valuation: $276m or $4.48 per diluted share
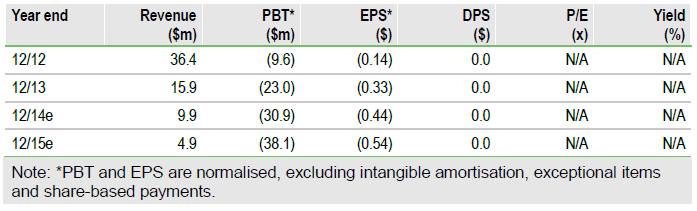
We value the company at $276m, a slight decrease from our previous $283m, based on slightly lower end-2014 cash. Our pipeline value remains at $217m, including $192m from tivantinib in second-line, c-Met high HCC and $25m for ARQ 087 and 092, both are in Phase I trials for solid tumours.
To Read the Entire Report Please Click on the pdf File Below