Alnylam Pharmaceuticals, Inc. (NASDAQ:ALNY) announced that the FDA has accepted a NDA for its lead candidate patisiran. The candidate is an investigational RNAi therapeutic targeting transthyretin (“TTR”) for the treatment of adults with hereditary ATTR amyloidosis (hATTR) with polyneuropathy. The FDA also granted priority review to patisiran and has set an action date of Aug 11, 2018.
Priority Review designation from the FDA is generally granted to drugs that have the potential to provide significant improvements in the safety and effectiveness of the treatment, prevention or diagnosis of a serious disease.
Patisiran has already been granted Fast Track Designation, Breakthrough Therapy Designation and an expanded Orphan Drug Designation for ATTR amyloidosis from the FDA.
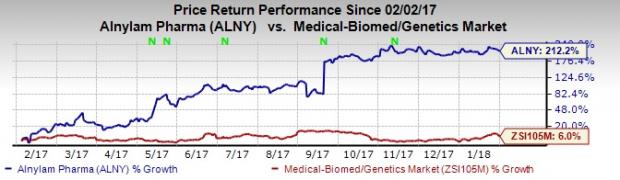
So far this year, shares of Alnylam have skyrocketed 212.2% compared with the industry’s gain of 6%.
In December 2017, the company completed the rolling submission of a NDA to the FDA for patisiran. The NDA filing was based on promising results from the APOLLO phase III study that met its primary as well as all secondary endpoints. The patients who were administered patisiran experienced significant improvement in quality of life compared to placebo.
The potential approval of patisiran is likely to be a huge boost and will be an important treatment option for the people suffering from this often fatal disease.
In January 2018, the European Medicines Agency ("EMA") accepted the Marketing Authorisation Application for patisiran, initiating their review. The same was submitted to the EMA along with Alnylam’s partner Sanofi (NYSE:SNY) in December 2017. In November 2017, the Committee for Medicinal Products for Human Use (“CHMP”) of the EMA granted an accelerated assessment for patisiran.
Alnylam will commercialize patisiran in the United States, Canada and Western Europe while Sanofi will commercialize the product in the rest of the world.
A potential approval of patisiran is likely to be a huge boost and will be an important treatment option for the people suffering from this fatal disease.
Zacks Rank & Stocks to Consider
Alnylam carries a Zacks Rank #3 (Hold).
Some better-ranked stocks from the health care space are XOMA Corporation (NASDAQ:XOMA) and Exelixis (NASDAQ:EXEL) . While XOMA sports a Zacks Rank #1 (Strong Buy), Exelixis carries a Zacks Rank #2 (Buy). You can see the complete list of today’s Zacks #1 Rank stocks here.
XOMA’s loss per share estimates have narrowed from 99 cents to 42 cents for 2018 in the last 60 days. The company pulled off a positive earnings surprise in one of the last four quarters, with an average beat of 47.92%. Share price of the company skyrocketed 624.9% over a year.
Exelixis’ earnings per share estimates have moved up from 72 cents to 77 cents for 2018 in the last 60 days. The company delivered positive earnings surprise in the last four quarters, with an average beat of 572.92%. Share price of the company surged 64.1% over a year.
Breaking News: Cryptocurrencies Now Bigger than Visa
The total market cap of all cryptos recently surpassed $700 billion – more than a 3,800% increase in the previous 12 months. They’re now bigger than Morgan Stanley (NYSE:MS), Goldman Sachs (NYSE:GS) and even Visa! The new asset class may expand even more rapidly in 2018 as new investors continue pouring in and Wall Street becomes increasingly involved.
Zacks has just named 4 companies that enable investors to take advantage of the explosive growth of cryptocurrencies via the stock market.
Click here to access these stocks >>
Sanofi (SNY): Free Stock Analysis Report
XOMA Corporation (XOMA): Free Stock Analysis Report
Alnylam Pharmaceuticals, Inc. (ALNY): Free Stock Analysis Report
Exelixis, Inc. (EXEL): Free Stock Analysis Report
Original post
Zacks Investment Research