Today, we will take a detailed look into a company that has an imminent catalyst, a number of important Phase II and Phase III data releases throughout 2013, and a product pipeline with potential to tap into a $17B market.
Agenus (AGEN) is a promising biotech company with two exciting platforms that focus on developing breakthrough immunotherapies for cancer and infectious diseases. Using their innovative Heat Shock Protein (HSP) and Saponin platforms, Agenus has developed a potentially lucrative pipeline of groundbreaking technologies and product candidates. Its products under development include QS-21 Stimulon adjuvant, which is in Phase III clinical trials for the treatment of malaria, melanoma, non-small cell lung cancer, and shingles, as well as for the treatment of various infectious diseases, multiple cancer types, and Alzheimers disease; and HerpV, a therapeutic vaccine candidate that is in Phase II clinical trial for the treatment of genital herpes.
Agenus' developmental pipeline focuses on therapies for some of the world's most disconcerting diseases. Their QS-21 Stimulon Adjuvant aims to strengthen the body's immune response to a vaccine's antigen and is being tested on Malaria, Melanoma, Shingles, Alzheimer's Disease, Genital Herpes, and Non-Small Cell Lung Cancer. Additionally, their Prophage series provides therapies for Renal Cell Carcinoma, newly diagnosed Glioma, and also recurrent Glioma. Aligned with powerful partners like GlaxoSmithKline (GSK) and Janssen, Agenus is currently under speculated and I believe investors have yet to valuate the potential of their pipeline.
Agenus' Platform and Pipeline:
- HSP Platform
Heat shock proteins are a type of protein with the purpose of regulating the timing and conformation (shape) of cells throughout the body. They are "chaperones" for cells in the sense that they bind to the peptides in a cell and form a complex, so that our immune system can recognize diseased cells. When a diseased cell is no longer able to function and dies, heat shock proteins are released from the cell and our body uses that as a signal to generate an immune response. To put it simply, Agenus takes advantage of this natural immune response by utilizing heat shock proteins as vaccines against cancers and infectious disease.
Agenus uses its heat shock protein platform for its Prophage Series of vaccines, which include Oncophage for Renal Cell Carminoma, G-100 for newly diagnosed Glioma, and G-200 for recurrent Glioma.
- Saponin Platform
The Saponin Platform consists of the QS-21 adjuvant, which is designed to strengthen the body's immune response to a vaccine's antigen, thus making it more effective. Saponin is actually extracted from the bark of the Quillaja saponaria tree and testing so far has shown that QS-21 is a safe adjuvant for a number of vaccine antigens.
According to the company's website, when QS-21 is incorporated into vaccines it has been shown to:
- Demonstrate efficacy via parenteral or mucosal administration routes
- Potentiate both prophylactic and therapeutic vaccines
- Potentiate immune responses to viral, bacterial, and parasitic antigens, including recombinant proteins, polysaccharide antigens, DNA vectors, conjugated antigens, multivalent vaccine formulations and notoriously weak antigens
- Exhibit a favorable safety profile
- Integrate easily with most excipients and formulations
- Synergize with other adjuvants as well as approaches using carrier proteins
From above, it is evident why a powerful pharmaceutical company such as GlaxoSmithKline is partnered with Agenus. QS-21 Stimulon Adjuvant can certainly help build market share for Glaxo's current drugs by increasing the efficacy of each. Thus, it is reasonable to speculate that other large pharmas could come knocking on the Agenus' door with partnering interest. Agenus already states that it has 10 other indications possible for this platform, so maybe they have been working on that already.
Some highlights from Agenus' QS-21 adjuvant include successful Phase III RTS, S: Malaria, Phase III MAGE A3: Stage IIIB/IIIC Melanoma, Phase III MAGE A3: Stage IB-111A NSCLC, and Phase III Herpes Zoster: Shingles. All of those indications are partnered with GSK. Additionally, AGEN is partnered with Janssen for Phase II ACC-001: Alzheimer's Disease.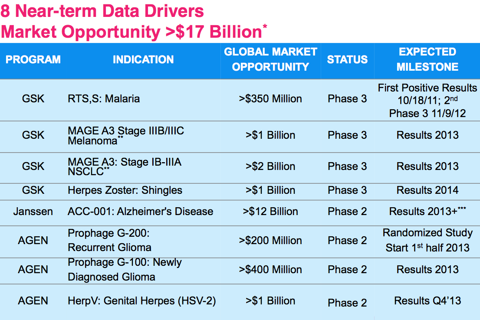
Together, the global market opportunity is over $17 billion for these products. The partnered products have the potential to bring Agenus over $100M a year royalties. The final 3 products on the chart are un partnered. If Agenus management executes its long term goals correctly, these 3 products can potentially bring the company over $1B in revenues. With a current market cap under $120 million, AGEN seems to be undervalued.
From the company's earnings call on April 24, Garo Armen - Chairman & CEO of Agenus remarks:
It is [QS-21] currently being studied in 17 clinical programs in development. Let me now switch gears and discuss the status of our internal development programs. As I mentioned earlier, our HerpV randomized double blind, multi-cancer Phase 2 trial in individuals infected with HerpV 2 is fully screened for enrolment. This puts us on track to report initial results during the fourth quarter of this year. I would like to note that HerpV is the most advanced therapeutic vaccine in clinical development today. This study is evaluating the efficacy of HerpV vaccine by measuring viral shedding before and after vaccination. In the study 65 participants will receive HerpV which contains QS-21 and a control group of 10 participants who will receive placebo. A booster injection will be given at six months after the initial treatment to evaluate both the potential of delayed effect as well as the durability of treatment effect. Key opinion leaders in the field have helped design the HerpV Phase II study and HSV-2 experts believe that a reduction in viral shedding, the driving force behind the spread of genital herpes is a key surrogate which could be predictive of clinical benefit defined by reduction of disease outbreaks. Among the unique attributes of HerpV is the fact that it contains 32 HSV-2 derived immunogenic antigens and was designed with the intent of treating a broad population of HSV-2 infected individuals.
We believe that if HerpV is ultimately shown to be a safe and effective product in late stage trials, it would represent a breakthrough in the treatment of herpes.
I believe Agenus has massive potential here to see multi-bagger gains in the future. Lately, we have seen a few small cap developmental pharmas have massive stock price runs based on their potential.
One such company that has seen multi-bagger gains in a short period of time is ACADIA Pharmaceuticals (ACAD).
ACADIA began 2013 trading around $4.60 a share. Just in the last few months, the stock has nearly tripled on both positive data for its lead drug pimavanserin, indicated for the treatment of Parkinson's disease psychosis (PDP), and allowance of an expedited New Drug Application (NDA) filing from the U.S. Food and Drug Administration (FDA).
PDP is an unmet need with a potentially huge market, and the company deserves its current speculation valuation. Agenus' propreitary product potential could be significantly larger than ACADIA'S in the long term. My opinion here is not meant as a competition between both companies, but rather to show that Agenus' potential has been under the radar.
Near term Catalyst:
Per the recent earnings conference call, Chief Executive Officer Garo Armen stated that Dr. Orin Bloch will speak on May 1st, 2013 at American Association of Neurological Surgeons annual scientific meeting about the results for the HSPPC-96 vaccination in patients with newly diagnosed Glioma. If positive, the speculated value of AGEN should be significantly higher, as the company moves forward into Phase III trials.
Afterwards, the company should be announcing the opening of its Phase III recurrent study in glioma patients. The National Cancer Institute (NCI) will be funding the study. The Phase III study is poised to be the largest ever funded by the NCI for brain tumors.
The glioma market is a lucrative one, with other companies ramping up clinical trials with the hope to grab their piece of the market.
Celldex Therapeutics (CLDX) is one company vying for a piece of the glioma market. Its drug rindopepimut is currently being evaluated in a study called ACT IV. ACT IV is a Phase III trial with enrollment currently underway. The trial is targeted to include 374 cancer patients by the end of enrollment. Celldex estimates enrollment to be completed by the end of this year.
Threshold Pharmaceuticals (THLD) also has a similar therapy for patients with reoccurring Glioblastoma termed TH-302. TH-302 is a Tumor Hypoxia-Targeted Drug, which is also combined with AVASTIN (Bevacizumab) in a Phase I/II trial to treat brain cancer patients. Threshold is in the early stage with this study, but is anticipating positive data from TH-302 for this very devastating and malignant disease.
Glioblastoma is an indication in which safe and effective therapies are very much needed.
Other Catalysts coming up this year:
Agenus has already reported positive Phase III results in late 2012 for the QS-21 Stimulon Adjuvant in GlaxoSmithKline's RTS,S for Malaria and has several other trials underway for additional indications. These catalysts include Phase III Mage A3 for Melanoma, and Phase III A3 for non-small cell lung cancer. Agenus is also partnered with GalaxoSmithKline for both of these indications. Furthermore, a Phase II HerpV trial for Genital Herpes is underway, with data due in the fourth quarter of 2013.
Speculating further into the future, Agenus has a Phase III trial for HZ/su for shingles with data due in 2014 and also 10 other undisclosed indications.
Financial Position:
According to Agenus' latest financial report, the company recently retired its outstanding $39 million 8.00% senior secured convertible notes due August 2014. These Notes were exchanged for $10 million in cash, 2,500,000 shares of common stock and a twenty percent revenue interest from QS-21 Stimulon partnered programs. In addition, the company entered into two separate $5 million debt transactions for $10 million total in notes plus 500,000 share warrants. Following these transactions, Agenus' total debt obligation outstanding is $10 million, down from $39 million.
Share Structure: 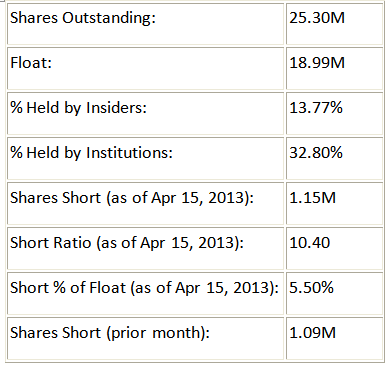
Agenus has a low float, which means that as traders gain interest in this stock, price appreciation should happen relatively quickly. Additionally, the small short interest shows that many traders realize the potential of this company and don't want to be caught short going into this data release. Also on a positive note, insiders hold a decent amount of the company, which is important in helping determine how much conviction the people running the company have in their own development.
Insider Transactions:
Garo Armen, the Chief Executive Officer of Agenus, acquired 250,277 shares on December 23, 2012 at a price of $4.33 per share. He has added additional shares as recently as March 31, 2013 and currently holds 927,400 shares total, worth over $4 million. The CEO purchasing a large amount is bullish and demonstrates that he is confident about the company going forward.
Charts and Indicators 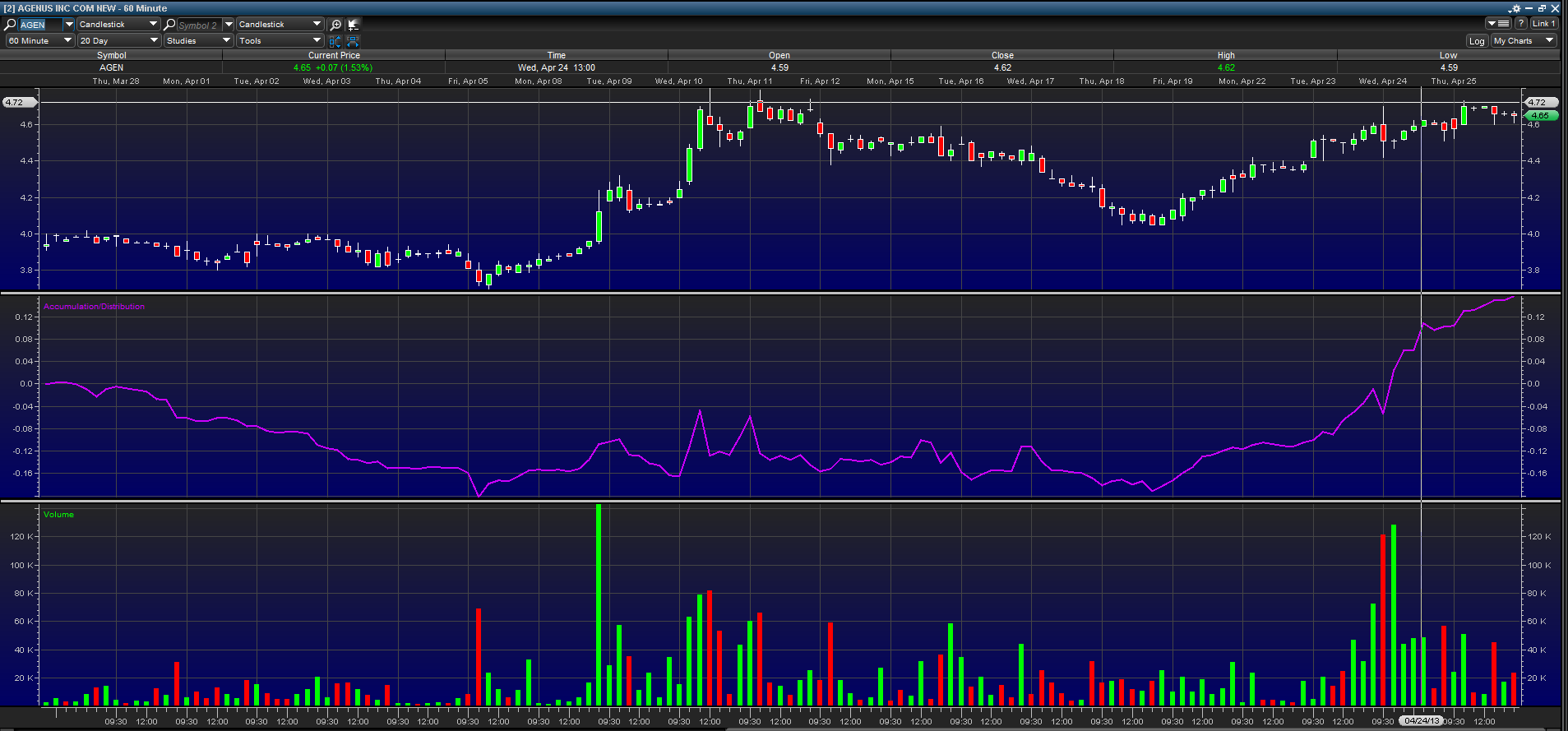
Taking a look at the above 60-minute chart over the last 20 trading sessions, we can see that traders have been accumulating Agenus. The stock price has been climbing steadily heading into the May 1 data presentation. The accumulation/distribution line has broken out and is in an uptrend with volume increasing.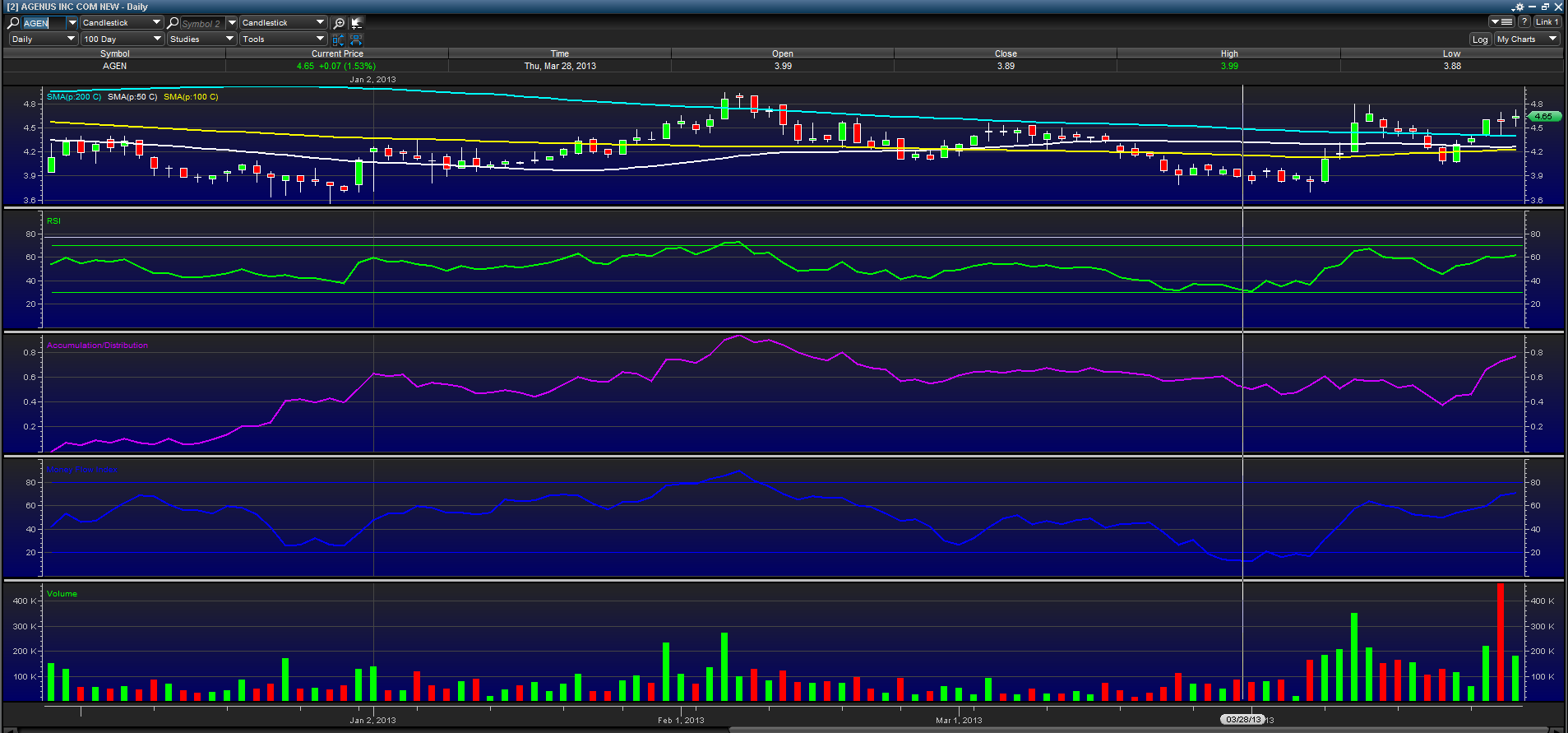
The above 100 day chart shows the stock is trading above its 50, 100 and 200 day moving averages, and the Money Flow Index and Relative Strength Index are in a current uptrend. All of these indicators signal bullish sentiment going forward.
In my opinion, AGEN is on the verge of breaking out of a longer term wedge and believe that share price will increase quickly in the days leading into the catalyst. Of course, if data release is positive after May 1st, we should also see a major increase in share price as investors speculate on AGEN's future.
Conclusion:
In my opinion, AGEN offers investors and traders an interesting opportunity in 2013. With several catalysts spread throughout the year, traders will be able to capitalize on the increased speculated value of the company. From an investing standpoint, AGEN has a promising pipeline of products and have aligned themselves with powerful partners. I believe AGEN is one of the better small-cap biotechnology companies on the market and shows great potential going forward.
My short term price target opinion is $5.50 per share heading into this Wednesday. On a good data release, I believe AGEN should reach $6.50 or so.
