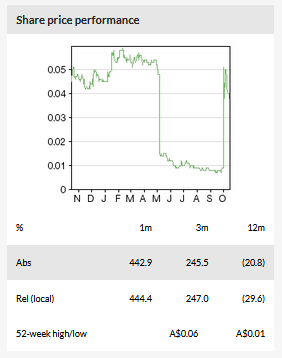
Actinogen Medical Ltd (ASX:ACW) share price has experienced a rather volatile period since May 2019, when it decreased c 70% following the disappointing Phase II XanADu trial results, only to rebound in October 2019 almost to pre-XanADu levels after preliminary results from another clinical trial XanaHES were reported. XanaHES was designed as a Phase I safety trial to test high doses of Xanamem, but a smart trial design (exploratory cognitive endpoints included) and well-timed initiation led to the right results being reported at the right time. Actinogen will still need to complete its R&D programme and pinpoint further R&D direction. Until then, our updated valuation stands at A$131m or A$0.12/share (vs A$0.17/share previously).
XanaHES study delivered more than expected
This Phase I study was designed to assess safety of higher doses of Xanamem (20mg and 30mg) and was meant to support the design of late-stage trials with Xanamem. In addition to standard safety endpoints, Actinogen included exploratory endpoints to assess the effect on cognition. Positive signals were obtained in three out of six domains in Cogstate Cognitive Test Battery. We note that the XanaHES study recruited healthy elderly volunteers, so the direct extrapolation of the data to diseases is not possible. XanaHES results serve more as a proof-of-concept that Xanamem can have a clinical effect. Whether this will translate into efficacy in certain CNS diseases can only be clarified in a clinical trial.
Pharmacologically active, but not effective at 10mg
The XanADu trial results reported in May 2019 and showed that Xanamem 10mg daily increased adrenocorticotropic (ACTH) and related hormones, which suggests it is pharmacologically active. However, this did not translate into clinical efficacy, as none of the co-primary endpoints (ADAS-Cog v14 and ADCOMS) or the secondary endpoints reached statistical significance. The key questions that remain unanswered include whether higher doses, longer treatment period and/or twice a day dosing rather than once a day would be effective. If positive, the full results from the XanaHES trial and the long-term toxicology data are the enabling datasets for longer-term treatment studies.
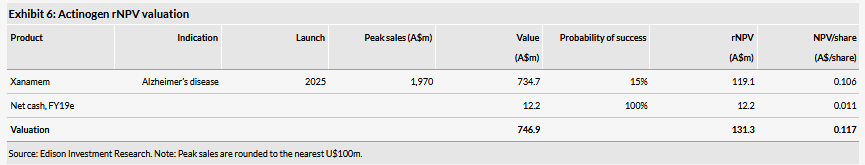
Valuation: A$131m or A$0.12/share
Our updated Actinogen valuation is A$131m or A$0.12/share. This is based on a risk-adjusted NPV model based on the Alzheimer’s disease (AD) indication, which we published in our initiation report. Following the new data publication, we believe the case that Xanamem is a cognition enhancer candidate still holds. We maintain our rNPV model based on AD indication but reduce the probability of success to 15% from 20% to account for the fact that the R&D strategy needs re-evaluation and we have deferred our modelled licensing deal by two years.
Business description
Actinogen Medical is an ASX-listed Australian biotech developing lead asset Xanamem, a specific 11beta-HSD1 inhibitor designed to treat cognitive impairment that occurs in chronic neurological and metabolic diseases.
XanaHES results: A positive surprise
We believe the surprise element came from the fact that even though the market focused almost solely on the Phase II XanADu trial, Actinogen had an active R&D programme with more clinical trials running (Exhibit 1):
Phase I target occupancy and homogenous binding studies. These studies have a novel method to prove that Xanamem inhibits 11β-HSD1. Using positron emission tomography (PET) and a radioactive tracer, it is possible to visualise the areas of the brain where Xanamem is active and calculate the target occupancy (Exhibit 5).
Phase I higher dose-safety study (XanaHES). This study was designed to assess safety of higher doses of Xanamem (20mg and 30mg) and the data were meant to support the design of the late-stage development of Xanamem (in AD or other indications) following the conclusion of the XanADu trial, where only one dose was used.
Pre-clinical long-term toxicology studies. This is a regulatory requirement for drug candidates that will be used as a chronic therapy. Animals can be dosed for months, so while not expensive, they can be time-consuming studies.
Actinogen initiated these supportive studies in 2018 following a successful fundraise. The rationale was that these will be complementary to the XanADu trial and will allow preparations for late-stage development of Xanamem in AD and other neuropsychiatric indications where the cognition worsens. It was the XanaHES study that delivered the surprise efficacy results and helped the share price rebound. This made it a timely decision to initiate the trial before the XanADu data were in.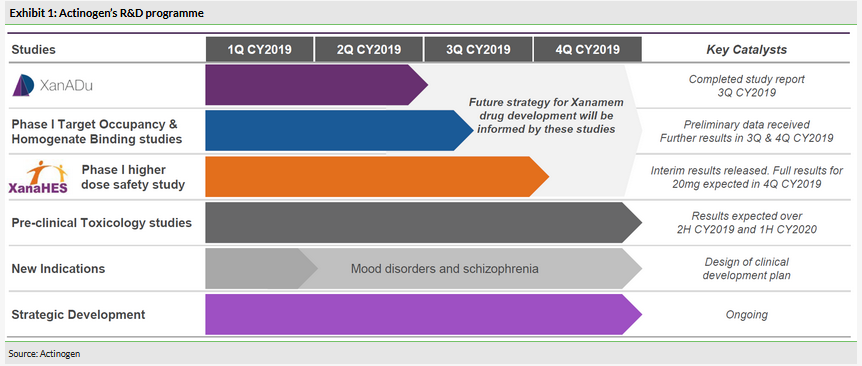
XanaHES results
On 1 October 2019, Actinogen announced results from its Phase I XanaHES trial (Xanamem in healthy elderly subjects). Primarily, this study was designed as a placebo-controlled trial to investigate higher doses of Xanamem (20mg and 30mg). Xanamem 10mg once a day dose was used in the XanADu trial. The XanaHES trial is split in two sequential cohorts testing daily doses of 20mg and 30mg. Each cohort includes 42 healthy elderly subjects (n=30 active arm, n=12 in placebo arm), who received Xanamem or placebo for 12 weeks. Actinogen announced results from the first cohort (20mg). Typical endpoints for these studies would focus around safety aspects of the drug, pharmacodynamics and pharmacokinetics. However, the company decided to include exploratory efficacy on cognition as well.
The decision was likely influenced by healthy volunteer studies done at the University of Edinburgh, which preceded the development of Xanamem. In the early 2000s, the researchers at the University of Edinburgh used carbenoxolone (a non-specific 11β-HSD inhibitor) in healthy volunteers (and type 2 diabetics) and were able to demonstrate an improvement in cognition (Sandeep et al., 2004). Early-stage development of Xanamem included dose-escalation studies, where the treatment period was shorter than 12 weeks and there was no control arm. So, these were not suitable to evaluate clinical efficacy endpoint. XanaHES, while primarily a safety study, was the first time healthy subjects received Xanamem for 12 weeks and the effects on cognition were evaluated.
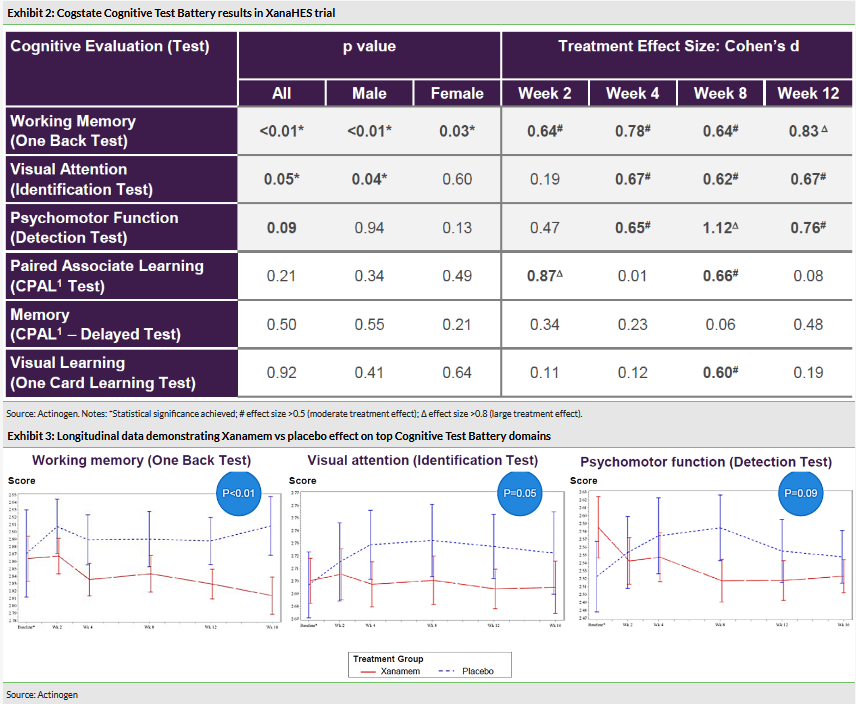
In the XanaHES trial Xanamem’s effect on cognition was measured using the standard Cogstate Cognitive Test Battery (a widely used tool to assess cognition when not necessarily focusing on AD). The Cogstate Battery evaluated six domains of cognition and the results of the trial showed that an improvement in two domains reached statistical significance (at 5% level) and trended towards significance in one other domain. More specifically:
The One Back Test demonstrated improvement in working memory with p1 of 0.83.
The Identification Test showed improvement in visual attention with p=0.05 and an effect size of 0.67.
The Detection Test showed a trend towards improved psychomotor function with p=0.09 and an effect size of 0.76.
The results confirmed a good safety profile with no reported serious adverse events and a statistically significant (p
Understanding XanADu trial results
The Phase II XanADu trial started in March 2017. It was a double-blind, placebo-controlled Phase II trial with mild AD patients (n=186), who received 10mg of Xanamem daily or placebo (1:1) for 12 weeks. Primary and secondary endpoints included gold-standard cognition assessment tools (ADAS-Cog v14 and ADCOMS as primary endpoints; RAVLT, CDR-SOB, MMSE, NPI and NTB, as secondary endpoints).
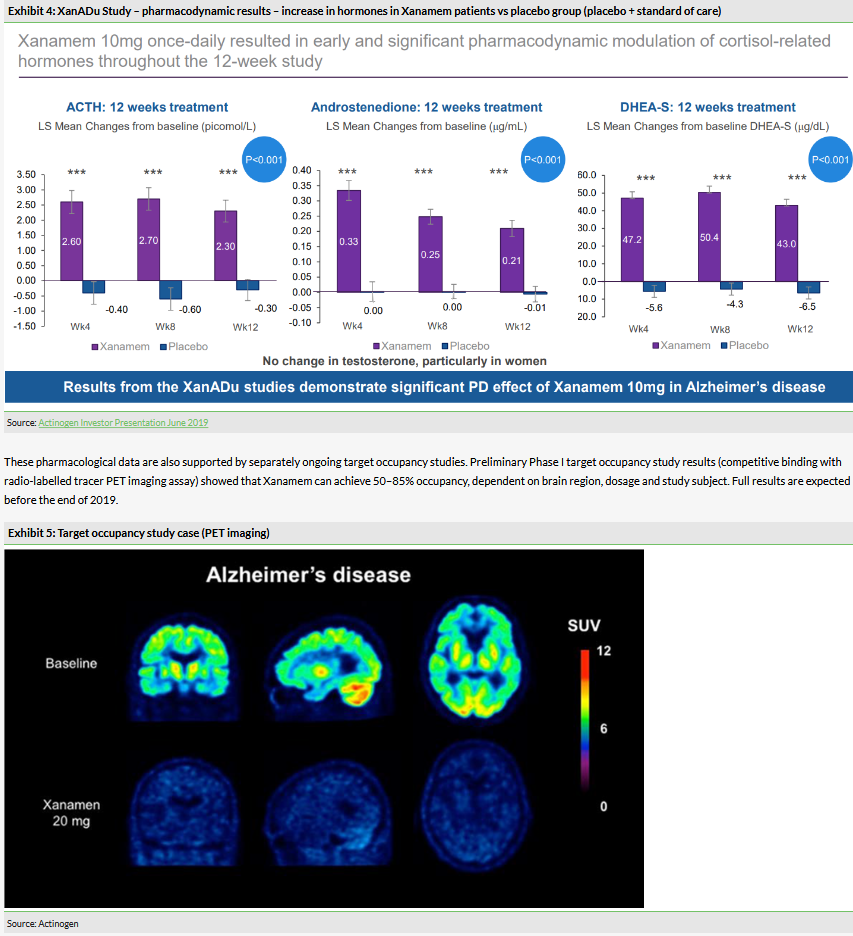
In May 2019, the results of the Phase IIb XanADu trial showed that Xanamem 10mg daily for 12 weeks was safe and pharmacologically effective, but the efficacy endpoints were missed. Xanamem 10mg daily did effectively inhibit cortisol production, as demonstrated by the expected increase in ACTH and related hormones. An increase in ACTH indicates that the hypothalamic–pituitary–adrenal axis is being affected. ACTH rises as a result of low cortisol and falls as a result of high cortisol. This suggests Xanamem is inhibiting 11β-HSD1.
These pharmacological data are also supported by separately ongoing target occupancy studies. Preliminary Phase I target occupancy study results (competitive binding with radio-labelled tracer PET imaging assay) showed that Xanamem can achieve 50–85% occupancy, dependent on brain region, dosage and study subject. Full results are expected before the end of 2019.
In our initiation report we described the available data and the rationale for Xanamem to be positioned as a cognitive enhancer. Given the new input from the XanADu trial, the key questions for us are:
Would a higher dose of Xanamem be effective? A higher dose makes sense from a safety perspective, as no significant adverse effects with 10mg (XanADu) and 20mg (XanaHES) emerged. In addition, higher doses than 10mg once daily (25mg, 35mg twice daily) were also safe for a shorter periods of treatment in the Phase I multiple ascending dose study. The upcoming results from 30mg dose cohort in XanaHES trial may provide some dose-response insights, which could be very supportive for the use of higher doses of Xanamem.
Was three months of treatment not enough to obtain an effect? The AD patients in the XanADu trial were treated for 12 weeks only due to several reasons. Such period of treatment was shown to be effective in the preclinical studies. A longer treatment period in humans requires long-term animal toxicology data, which is a time-consuming undertaking (studies are ongoing and the results are expected in H219/H120). We believe the fact that none of the endpoints showed statistical significance in the XanADu trial does not negate the question of whether a longer period treatment could be effective. AD is usually a very slow-progressing disease. To detect the slowing of the progression (disease modifying effect) or even symptomatic improvement could still require a long period of treatment.
Next steps
Even before the XanADu trial results were released, Actinogen was considering and performed an extensive literature review to understand other potential indications for Xanamem. Cognition impairment in mood and psychotic disorders such as bipolar disorder and schizophrenia were chosen as top priorities beyond AD. During the analyst conference call, management discussed that AD is the primary indication, but other the available options include cognitive impairment in neuropsychiatric disorders, such as mood disorders, eg bipolar disorder and schizophrenia. The final decision on specific clinical trials will depend on the full analysis of the XanaHES and XanADu trials, as well as the target occupancy studies. We note that the XanaHES study recruited healthy elderly volunteers, so the direct extrapolation of the data to disease is not possible. XanaHES results serve more as a proof-of-concept that Xanamem can have a clinical effect. Whether this will translate into efficacy in certain CNS diseases can only be clarified in a clinical trial. The management reiterated its commitment to advancing Xanamem’s development as soon as possible. Depending on the funding situation, the company could be ready to initiate new clinical trials next year, as the key studies in the current R&D programme will be completed by then.
Financials and valuation
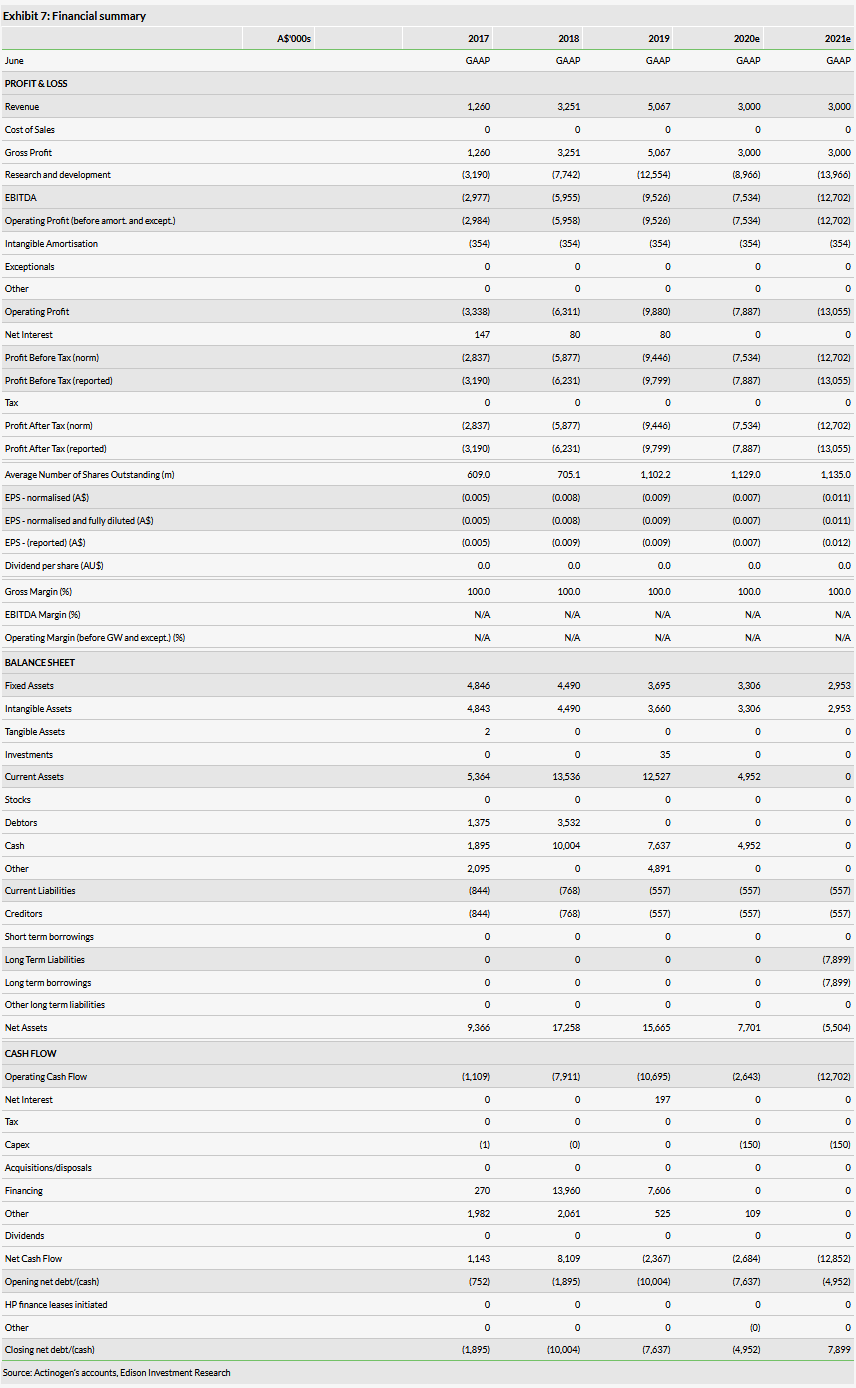
Actinogen’s operating FY19 results (end of June 2019) were largely in line with our expectations. Cash was A$7.6m at end of June 2019 (end FY19). This and R&D tax rebates (A$4.6m expected in calendar H219) should be sufficient to finish the ongoing R&D programme and cover corporate expenses to the end of calendar year 2020. By then, Actinogen will have finished the remaining clinical studies and will have all the inputs to decide further development of Xanamem. Subsequent studies will require additional funding. Actinogen indicated that it is investigating all potential financing sources including non-dilutive funding. Actinogen also mentioned that it is in constant dialog with pharma companies that could be potential partners in the future.
We developed a detailed risk-adjusted NPV model to assess Xanamem’s opportunity in AD in our initiation report, which was based on a number of assumptions. Since then, Actinogen reported data from two different clinical trials with both negative and positive surprises. From a broad point of view, the case that Xanamem is a cognition enhancer candidate still holds; however, it will take a bit more time until Actinogen will propose further concrete R&D plans. Because AD is still one of the top priorities and, due to a lack of any more concrete future R&D plans, we maintain our rNPV model based on the AD indication, which we believe still captures the value of Actinogen. We only made two changes. We reduced the success probability to 15% from 20% to account for the fact that the R&D strategy needs re-evaluation. Recently published updates on historical success rates show that assets in CNS in Phase II have success to reach the market rates in the range of 14-24% (Wong et al., 2019; BIO, 2016). We also deferred the licensing deal we had assumed and modelled using benchmarks by two years (from 2021 to 2023) mainly because such potentially substantial cash flows in the near term can significantly affect the rNPV. So, the postponement is a more conservative approach and reduces the model’s sensitivity to the licensing deal. Our updated Actinogen valuation is A$131m or A$0.12/share.